Tumor-infiltrating lymphocytes (TILs) are effective in the treatment of metastatic melanoma (MM), but toxicity limits its application. TILT-123 (igrelimogene litadenorepvec) is an oncolytic adenovirus producing interleukin-2 (IL-2) and tumor necrosis factor (TNF) upon replication. In this phase 1 trial, 17 patients with metastatic checkpoint inhibitor-resistant melanoma are treated with TILT-123 and TILs without preconditioning chemotherapy or postconditioning IL-2. The treatment is safe and feasible. According to computed tomography (CT), the objective response rate is 11.7% (2/17) and disease control is observed in 35% (6/17), including a partial response lasting >8 months and a durable complete response in a mucosal melanoma patient. According to positron emission tomography (PET), disease control is observed in 7/15 (47%) with minor or partial responses in 4/15 (27%). In the initial TILT-123 monotherapy phase of the trial, disease control is observed in 6/17 (35%) and 10/16 (63%) in CT and PET, respectively. The study demonstrates good tolerability and preliminary efficacy.
Copyright © 2025 The Authors. Published by Elsevier Inc. All rights reserved.
Product Citations: 21
In Cell Reports Medicine on 18 March 2025 by Monberg, T. J., Pakola, S. A., et al.
-
Cancer Research
In IScience on 19 April 2024 by Sjøgren, T., Islam, S., et al.
Immune tolerance fails in autoimmune polyendocrine syndrome type 1 (APS-1) because of AIRE mutations. We have used single cell transcriptomics to characterize regulatory T cells (Tregs) sorted directly from blood and from in vitro expanded Tregs in APS-1 patients compared to healthy controls. We revealed only CD52 and LTB (down) and TXNIP (up) as consistently differentially expressed genes in the datasets. There were furthermore no large differences of the TCR-repertoire of expanded Tregs between the cohorts, but unique patients showed a more restricted use of specific clonotypes. We also found that in vitro expanded Tregs from APS-1 patients had similar suppressive capacity as controls in co-culture assays, despite expanding faster and having more exhausted cells. Our results suggest that APS-1 patients do not have intrinsic defects in their Treg functionality, and that their Tregs can be expanded ex vivo for potential therapeutic applications.
© 2024 The Author(s).
-
Homo sapiens (Human)
-
Cardiovascular biology
-
Immunology and Microbiology
In IScience on 20 January 2023 by Touizer, E., Alrubayyi, A., et al.
We assessed a cohort of people living with human immunodeficiency virus (PLWH) (n = 110) and HIV negative controls (n = 64) after 1, 2 or 3 SARS-CoV-2 vaccine doses. At all timepoints, PLWH had significantly lower neutralizing antibody (nAb) titers than HIV-negative controls. We also observed a delayed development of neutralization in PLWH that was underpinned by a reduced frequency of spike-specific memory B cells (MBCs). Improved neutralization breadth was seen against the Omicron variant (BA.1) after the third vaccine dose in PLWH but lower nAb responses persisted and were associated with global MBC dysfunction. In contrast, SARS-CoV-2 vaccination induced robust T cell responses that cross-recognized variants in PLWH. Strikingly, individuals with low or absent neutralization had detectable functional T cell responses. These PLWH had reduced numbers of circulating T follicular helper cells and an enriched population of CXCR3+CD127+CD8+T cells after two doses of SARS-CoV-2 vaccination.
© 2023 The Authors.
-
COVID-19
-
Immunology and Microbiology
In Nutrients on 12 January 2023 by de Lange, I. H., van Gorp, C., et al.
Many whey proteins, peptides and protein-derived amino acids have been suggested to improve gut health through their anti-oxidant, anti-microbial, barrier-protective and immune-modulating effects. Interestingly, although the degree of hydrolysis influences peptide composition and, thereby, biological function, this important aspect is often overlooked. In the current study, we aimed to investigate the effects of whey protein fractions with different degrees of enzymatic hydrolysis on the intestinal epithelium in health and disease with a novel 2D human intestinal organoid (HIO) monolayer model. In addition, we aimed to assess the anti-microbial activity and immune effects of the whey protein fractions. Human intestinal organoids were cultured from adult small intestines, and a model enabling apical administration of nutritional components during hypoxia-induced intestinal inflammation and normoxia (control) in crypt-like and villus-like HIO was established. Subsequently, the potential beneficial effects of whey protein isolate (WPI) and two whey protein hydrolysates with a 27.7% degree of hydrolysis (DH28) and a 50.9% degree of hydrolysis (DH51) were assessed. In addition, possible immune modulatory effects on human peripheral immune cells and anti-microbial activity on four microbial strains of the whey protein fractions were investigated. Exposure to DH28 prevented paracellular barrier loss of crypt-like HIO following hypoxia-induced intestinal inflammation with a concomitant decrease in hypoxia inducible factor 1 alpha (HIF1α) mRNA expression. WPI increased Treg numbers and Treg expression of cluster of differentiation 25 (CD25) and CD69 and reduced CD4+ T cell proliferation, whereas no anti-microbial effects were observed. The observed biological effects were differentially mediated by diverse whey protein fractions, indicating that (degree of) hydrolysis influences their biological effects. Moreover, these new insights may provide opportunities to improve immune tolerance and promote intestinal health.
-
FC/FACS
-
Homo sapiens (Human)
In Life (Basel, Switzerland) on 2 May 2022 by Kalavska, K., Sestakova, Z., et al.
The tumor microenvironment (TME) and the host inflammatory response are closely interconnected. The interplay between systemic inflammation and the local immune response may influence tumor development and progression in various types of cancer. The systemic immune-inflammation index (SII) represents a prognostic marker for germ cell tumors (GCTs). The aim of the present study was to detect specific immune cell subpopulation changes which were associated with the SII level in chemotherapy-naïve GCT patients. In total, 51 GCT patients, prior to cisplatin-based chemotherapy, were included in the present study. Immunophenotyping of peripheral blood leukocyte subpopulations was performed using flow cytometry. The SII level was correlated with the percentage of various leukocyte subpopulations. The obtained results demonstrated that SII levels above the cut-off value of SII ≥ 1003 were associated with higher neutrophil percentages. An inverse correlation was found between the SII and the peripheral lymphocyte percentage that logically reflects the calculations of the SII index. Furthermore, the presented data also showed that in the lymphocyte subpopulation, the association with the SII was driven by T-cell subpopulations. In innate immunity-cell subpopulations, we observed a correlation between SII level and neutrophils as well as associations with eosinophil, basophil, natural killer cell and dendritic cell percentages. We suppose that the described interactions represent a manifestation of cancer-induced immune suppression. The results of the present study contribute to the elucidation of the interrelationship between tumor cells and the innate/adaptive immune system of the host.
-
FC/FACS
-
Homo sapiens (Human)
-
Cancer Research
-
Immunology and Microbiology
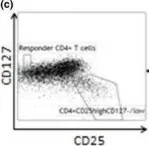
In J Intern Med on 1 January 2016 by Hasib, L., Lundberg, A. K., et al.
Fig.1.C

-
FC/FACS
-
Collected and cropped from J Intern Med by CiteAb, provided under a CC-BY license
Image 1 of 1