Adaptive processes of the innate immune system, known as trained immunity (TI), are critical to human health and disease, yet they have not been systematically investigated downstream of antiviral sensing. Here, we elucidate the potential of the antiviral cytosolic RNA receptor retinoic acid-inducible gene I (RIG-I) to train, prime and tolerize the innate immune system. Using a specific RIG-I agonist, we observed that repetitive stimulation enhanced interferon-stimulated gene (ISG) and pro-inflammatory cytokine induction in human primary monocytes, epithelial cells and fibroblasts and afforded non-specific antiviral protection. RNA sequencing revealed broad, cell type-specific transcriptional changes, indicative of priming of ISGs and training of the NFκB pathway, without measurable tolerization, while ATAC sequencing in monocytes demonstrated chromatin remodeling and enhanced accessibility of key transcription factor-binding motifs such as STAT1. Moreover, while STAT1 signaling was critically required, it was not sufficient to recapitulate RIG-I induced TI. Altogether, our data demonstrate that RIG-I-mediated TI promotes an immunologically alert state with important implications for host defense and the application of RIG-I ligands in anti-infective and anti-tumoral therapies. One Sentence Summary RIG-I activation trains and primes innate immune response at the cellular level, affording non-specific immune protection by immune and non-immune cells.
Product Citations: 21
RIG-I activation primes and trains innate antiviral immune memory
Preprint on BioRxiv : the Preprint Server for Biology on 28 October 2022 by Adamson, M. S., Nesic, S., et al.
-
FC/FACS
-
Immunology and Microbiology
In Vaccines on 23 June 2022 by Pancisi, E., Granato, A. M., et al.
Advanced therapy medical products (ATMPs) are rapidly growing as innovative medicines for the treatment of several diseases. Hence, the role of quality analytical tests to ensure consistent product safety and quality has become highly relevant. Several clinical trials involving dendritic cell (DC)-based vaccines for cancer treatment are ongoing at our institute. The DC-based vaccine is prepared via CD14+ monocyte differentiation. A fresh dose of 10 million DCs is administered to the patient, while the remaining DCs are aliquoted, frozen, and stored in nitrogen vapor for subsequent treatment doses. To evaluate the maintenance of quality parameters and to establish a shelf life of frozen vaccine aliquots, a stability program was developed. Several parameters of the DC final product at 0, 6, 12, 18, and 24 months were evaluated. Our results reveal that after 24 months of storage in nitrogen vapor, the cell viability is in a range between 82% and 99%, the expression of maturation markers remains inside the criteria for batch release, the sterility tests are compliant, and the cell costimulatory capacity unchanged. Thus, the data collected demonstrate that freezing and thawing do not perturb the DC vaccine product maintaining over time its functional and quality characteristics.
-
FC/FACS
-
Cancer Research
-
Immunology and Microbiology
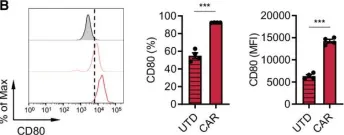
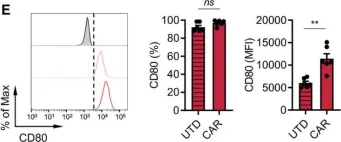
PD-L1 blockade restores CAR T cell activity through IFN-γ-regulation of CD163+ M2 macrophages.
In Journal for Immunotherapy of Cancer on 1 June 2022 by Yamaguchi, Y., Gibson, J., et al.
The immune suppressive tumor microenvironment (TME) that inhibits T cell infiltration, survival, and antitumor activity has posed a major challenge for developing effective immunotherapies for solid tumors. Chimeric antigen receptor (CAR)-engineered T cell therapy has shown unprecedented clinical response in treating patients with hematological malignancies, and intense investigation is underway to achieve similar responses with solid tumors. Immunologically cold tumors, including prostate cancers, are often infiltrated with abundant tumor-associated macrophages (TAMs), and infiltration of CD163+ M2 macrophages correlates with tumor progression and poor responses to immunotherapy. However, the impact of TAMs on CAR T cell activity alone and in combination with TME immunomodulators is unclear.
To model this in vitro, we utilized a novel co-culture system with tumor cells, CAR T cells, and polarized M1 or M2 macrophages from CD14+ peripheral blood mononuclear cells collected from healthy human donors. Tumor cell killing, T cell activation and proliferation, and macrophage phenotypes were evaluated by flow cytometry, cytokine production, RNA sequencing, and functional blockade of signaling pathways using antibodies and small molecule inhibitors. We also evaluated the TME in humanized mice following CAR T cell therapy for validation of our in vitro findings.
We observed inhibition of CAR T cell activity with the presence of M2 macrophages, but not M1 macrophages, coinciding with a robust induction of programmed death ligand-1 (PD-L1) in M2 macrophages. We observed similar PD-L1 expression in TAMs following CAR T cell therapy in the TME of humanized mice. PD-L1, but not programmed cell death protein-1, blockade in combination with CAR T cell therapy altered phenotypes to more M1-like subsets and led to loss of CD163+ M2 macrophages via interferon-γ signaling, resulting in improved antitumor activity of CAR T cells.
This study reveals an alternative mechanism by which the combination of CAR T cells and immune checkpoint blockade modulates the immune landscape of solid tumors to enhance therapeutic efficacy of CAR T cells.
© Author(s) (or their employer(s)) 2022. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
-
FC/FACS
-
Immunology and Microbiology
In Frontiers in Immunology on 1 February 2022 by Ceglia, V., Zurawski, S., et al.
CD40 is a potent activating receptor expressed on antigen-presenting cells (APCs) of the immune system. CD40 regulates many aspects of B and T cell immunity via interaction with CD40L expressed on activated T cells. Targeting antigens to CD40 via agonistic anti-CD40 antibody fusions promotes both humoral and cellular immunity, but current anti-CD40 antibody-antigen vaccine prototypes require co-adjuvant administration for significant in vivo efficacy. This may be a consequence of dulling of anti-CD40 agonist activity via antigen fusion. We previously demonstrated that direct fusion of CD40L to anti-CD40 antibodies confers superagonist properties. Here we show that anti-CD40-CD40L-antigen fusion constructs retain strong agonist activity, particularly for activation of dendritic cells (DCs). Therefore, we tested anti-CD40-CD40L antibody fused to antigens for eliciting immune responses in vitro and in vivo. In PBMC cultures from HIV-1-infected donors, anti-CD40-CD40L fused to HIV-1 antigens preferentially expanded HIV-1-specific CD8+ T cells versus CD4+ T cells compared to analogous anti-CD40-antigen constructs. In normal donors, anti-CD40-CD40L-mediated delivery of Influenza M1 protein elicited M1-specific T cell expansion at lower doses compared to anti-CD40-mediated delivery. Also, on human myeloid-derived dendritic cells, anti-CD40-CD40L-melanoma gp100 peptide induced more sustained Class I antigen presentation compared to anti-CD40-gp100 peptide. In human CD40 transgenic mice, anti-CD40-CD40L-HIV-1 gp140 administered without adjuvant elicited superior antibody responses compared to anti-CD40-gp140 antigen without fused CD40L. In human CD40 mice, compared to the anti-CD40 vehicle, anti-CD40-CD40L delivery of Eα 52-68 peptide elicited proliferating of TCR I-Eα 52-68 CD4+ T cells producing cytokine IFNγ. Also, compared to controls, only anti-CD40-CD40L-Cyclin D1 vaccination of human CD40 mice reduced implanted EO771.LMB breast tumor cell growth. These data demonstrate that human CD40-CD40L antibody fused to antigens maintains highly agonistic activity and generates immune responses distinct from existing low agonist anti-CD40 targeting formats. These advantages were in vitro skewing responses towards CD8+ T cells, increased efficacy at low doses, and longevity of MHC Class I peptide display; and in mouse models, a more robust humoral response, more activated CD4+ T cells, and control of tumor growth. Thus, the anti-CD40-CD40L format offers an alternate DC-targeting platform with unique properties, including intrinsic adjuvant activity.
Copyright © 2022 Ceglia, Zurawski, Montes, Kroll, Bouteau, Wang, Ellis, Igyártó, Lévy and Zurawski.
-
FC/FACS
-
Immunology and Microbiology
PD-L1 blockade restores CAR T cell activity through IFNγ-regulation of CD163+ macrophages
Preprint on BioRxiv : the Preprint Server for Biology on 28 January 2022 by Yamaguchi, Y., Gibson, J., et al.
h4>Background/h4> The immune suppressive tumor microenvironment (TME) that inhibits T cell infiltration, survival, and anti-tumor activity has posed a major challenge for developing effective immunotherapies for solid tumors. Chimeric antigen receptor (CAR)-engineered T cell therapy has shown unprecedented clinical response in treating patients with hematological malignancies, and intense investigation is underway to achieve similar responses with solid tumors. Immunologically cold tumors, including prostate cancers, are often infiltrated with abundant tumor-associated macrophages (TAMs), and infiltration of CD163 + M2 macrophages correlates with tumor progression and poor responses to immunotherapy. However, the impact of TAMs on CAR T cell activity alone and in combination with TME immunomodulators is unclear. h4>Methods/h4> To model this in vitro , we utilized a novel co-culture system with tumor cells, CAR T cells, and polarized M1 or M2 macrophages from CD14 + PBMCs collected from healthy human donors. Tumor cell killing, T cell activation and proliferation, and macrophage phenotypes were evaluated by flow cytometry, cytokine production, RNA sequencing, and functional blockade of signaling pathways using antibodies and small molecule inhibitors. We also evaluated the TME in humanized mice following CAR T cell therapy for validation of our in vitro findings. h4>Results/h4> We observed inhibition of CAR T cell activity with the presence of M2 macrophages, but not M1 macrophages, coinciding with a robust induction of PD-L1 in M2 macrophages. We observed similar PD-L1 expression in TAMs following CAR T cell therapy in the TME of humanized mice. PD-L1, but not PD-1, blockade in combination with CAR T cell therapy altered phenotypes to more M1-like subsets and led to loss of CD163 + M2 macrophages via IFNγ signaling, resulting in improved anti-tumor activity of CAR T cells. h4>Conclusion/h4> This study reveals an alternative mechanism by which the combination of CAR T cells and immune checkpoint blockade modulates the immune landscape of solid tumors to enhance therapeutic efficacy of CAR T cells.
-
Immunology and Microbiology
In J Immunother Cancer on 1 June 2022 by Yamaguchi, Y., Gibson, J., et al.
Fig.2.B

-
FC/FACS
-
Collected and cropped from J Immunother Cancer by CiteAb, provided under a CC-BY license
Image 1 of 2
In J Immunother Cancer on 1 June 2022 by Yamaguchi, Y., Gibson, J., et al.
Fig.2.E

-
FC/FACS
-
Collected and cropped from J Immunother Cancer by CiteAb, provided under a CC-BY license
Image 1 of 2