Understanding the evolution of the B cell response to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) variants is fundamental to design the next generation of vaccines and therapeutics. We longitudinally analyze at the single-cell level almost 900 neutralizing human monoclonal antibodies (nAbs) isolated from vaccinated people and from individuals with hybrid and super hybrid immunity (SH), developed after three mRNA vaccine doses and two breakthrough infections. The most potent neutralization and Fc functions against highly mutated variants belong to the SH cohort. Repertoire analysis shows that the original Wuhan antigenic sin drives the convergent expansion of the same B cell germlines in vaccinated and SH cohorts. Only Omicron breakthrough infections expand previously unseen germ lines and generate broadly nAbs by restoring IGHV3-53/3-66 germ lines. Our analyses find that B cells initially expanded by the original antigenic sin continue to play a fundamental role in the evolution of the immune response toward an evolving virus.
Copyright © 2024 The Author(s). Published by Elsevier Inc. All rights reserved.
Product Citations: 20
In Cell Reports on 24 September 2024 by Paciello, I., Pierleoni, G., et al.
-
COVID-19
-
Immunology and Microbiology
B cell maturation restored ancestral germlines to control Omicron BA.2.86
Preprint on BioRxiv : the Preprint Server for Biology on 4 March 2024 by Paciello, I., Pierleoni, G., et al.
ABSTRACT The unceasing interplay between SARS-CoV-2 and the human immune system has led to a continuous maturation of the virus and B cell response providing an opportunity to track their evolution in real time. We longitudinally analyzed the functional activity of almost 1,000 neutralizing human monoclonal antibodies (nAbs) isolated from vaccinated people, and from individuals with hybrid and super hybrid immunity (SH), developed after three mRNA vaccine doses and two breakthrough infections. The most potent neutralization and Fc functions against highly mutated variants, including BA.2.86, were found in the SH cohort. Despite different priming, epitope mapping revealed a convergent maturation of the functional antibody response. Neutralization was mainly driven by Class 1/2 nAbs while Fc functions were induced by Class 3/4 antibodies. Remarkably, broad neutralization was mediated by restored IGHV3-53/3-66 B cell germlines which, after heterogenous exposure to SARS-CoV-2 S proteins, increased their level of somatic hypermutations. Our study shows the resilience of the human immune system which restored previously expanded germlines and activated naïve B cells to broaden the antibody repertoire of antibodies to control future SARS-CoV-2 variants.
-
Immunology and Microbiology
Preprint on BioRxiv : the Preprint Server for Biology on 8 December 2023 by Troisi, M., Fabbrini, M., et al.
ABSTRACT Gonococcus (Gc), a bacterium resistant to most antibiotics causing more than 80 million cases of gonorrhea annually, is a WHO high priority pathogen. Recently, vaccine development prospects were boosted by reports that licensed meningococcus serogroup B (MenB) vaccines provided partial protection against Gc infection. To determine antigens responsible for cross-protection, memory B cells from 4CMenB vaccinated volunteers were single-cell sorted to identify antibodies that kill Gc in a bactericidal assay. Nine different antibodies, all deriving from the IGHV4-34 germline carrying unusually long HCDR3s, recognized the PorB protein, four recognized the lipooligosaccharide (LOS), and four unknown antigens. One of the PorB antibodies, tested in vivo, provided protection from Gc infection. The identification of PorB and LOS as key antigens of gonococcal and meningococcal immunity provides a mechanistic explanation of the cross-protection observed in the clinic and shows that isolating human monoclonal antibodies from vaccinees can be instrumental for bacterial antigen discovery.
In IScience on 20 October 2023 by Guerra, D., Beaumont, T., et al.
The pandemic caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has remained a medical threat due to the evolution of multiple variants that acquire resistance to vaccines and prior infection. Therefore, it is imperative to discover monoclonal antibodies (mAbs) that neutralize a broad range of SARS-CoV-2 variants. A stabilized spike glycoprotein was used to enrich antigen-specific B cells from an individual with a primary Gamma variant infection. Five mAbs selected from those B cells showed considerable neutralizing potency against multiple variants, with COVA309-35 being the most potent against the autologous virus, as well as Omicron BA.1 and BA.2, and COVA309-22 having binding and neutralization activity against Omicron BA.4/5, BQ.1.1, and XBB.1. When combining the COVA309 mAbs as cocktails or bispecific antibodies, the breadth and potency were improved. In addition, the mechanism of cross-neutralization of the COVA309 mAbs was elucidated by structural analysis. Altogether these data indicate that a Gamma-infected individual can develop broadly neutralizing antibodies.
© 2023 The Authors.
-
COVID-19
In Nature Communications on 4 January 2023 by Andreano, E., Paciello, I., et al.
The continuous evolution of SARS-CoV-2 generated highly mutated variants able to escape natural and vaccine-induced primary immunity. The administration of a third mRNA vaccine dose induces a secondary response with increased protection. Here we investigate the longitudinal evolution of the neutralizing antibody response in four donors after three mRNA doses at single-cell level. We sorted 4100 spike protein specific memory B cells identifying 350 neutralizing antibodies. The third dose increases the antibody neutralization potency and breadth against all SARS-CoV-2 variants as observed with hybrid immunity. However, the B cell repertoire generating this response is different. The increases of neutralizing antibody responses is largely due to the expansion of B cell germlines poorly represented after two doses, and the reduction of germlines predominant after primary immunization. Our data show that different immunization regimens induce specific molecular signatures which should be considered while designing new vaccines and immunization strategies.
© 2023. The Author(s).
-
FC/FACS
-
COVID-19
-
Genetics
-
Immunology and Microbiology
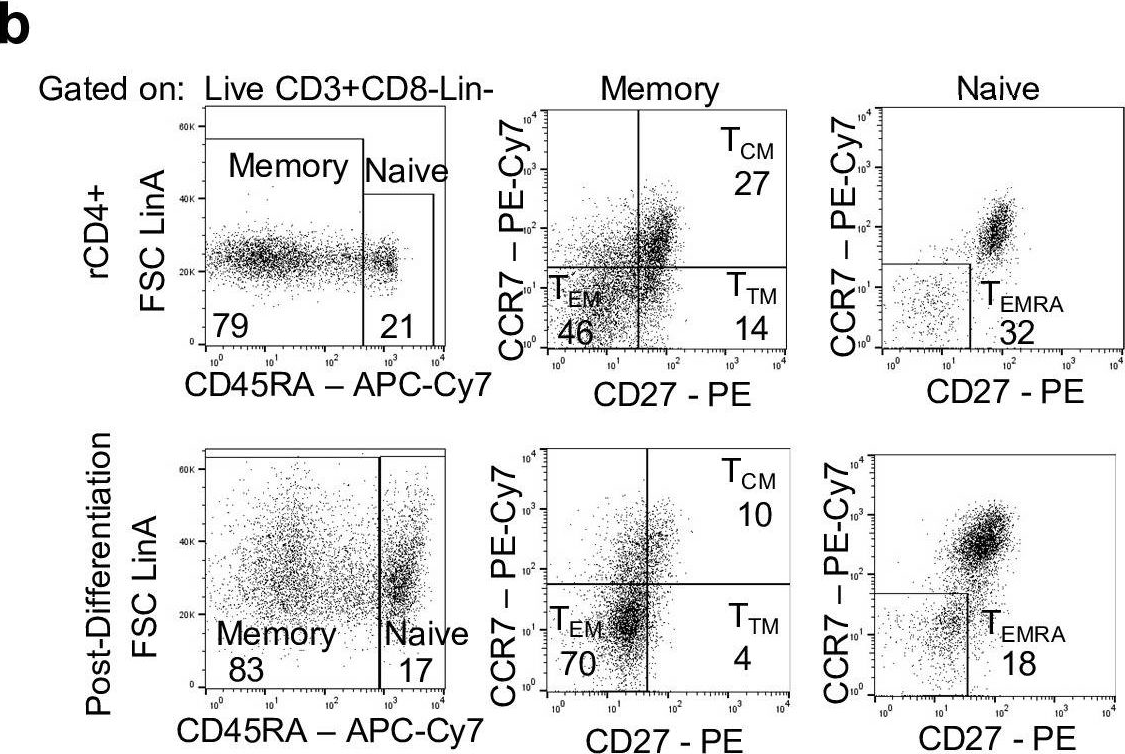
In PLoS Pathog on 1 October 2019 by Wonderlich, E. R., Subramanian, K., et al.
Fig.1.B
-
FC/FACS
-
Homo sapiens (Human)
Collected and cropped from PLoS Pathog by CiteAb, provided under a CC-BY license
Image 1 of 1