Human cytomegalovirus (HCMV) is a relevant pathogen, especially for individuals with impaired immunity. Harnessing potent immune antagonists, HCMV circumvents sterile immunity. Given that HCMV prevents the upregulation of human leukocyte antigen (HLA)-DP and HLA-DR, we screened a library of HCMV genes by co-expression with the HLA class II (HLA-II)-inducing transcription coordinator class II transactivator (CIITA). We identified the latency regulator pUS28 as an interaction factor and potent viral antagonist of CIITA-driven expression of CD74, HLA-DR, HLA-DM, HLA-DQ, and HLA-DP. Both wt-pUS28 and a mutant incapable of inducing G protein-coupled signaling (R129A), but not a mutant lacking the C-terminus, drastically reduced the CIITA protein abundance post-transcriptionally. While control CD4 + T cells from HCMV-seropositive individuals vigorously responded to CIITA-expressing cells decorated with HCMV antigens, pUS28 expression was sufficient to inhibit HLA-II induction and immune recognition by HCMV-specific CD4 + T cells. Our data uncover pUS28 to be employed by HCMV to evade HLA-II-mediated recognition by CD4 + T cells.
© 2025, Maassen et al.
Product Citations: 101
The human cytomegalovirus-encoded pUS28 antagonizes CD4+ T cell recognition by targeting CIITA.
In eLife on 3 July 2025 by Maassen, F., Le-Trilling, V. T. K., et al.
-
Immunology and Microbiology
Preprint on BioRxiv : the Preprint Server for Biology on 20 April 2025 by Ormhøj, M., Munk, K. K., et al.
Adoptive cell therapy (ACT) has shown promising results in cancer treatment, however, achieving effective ex vivo expansion of potent, functionally active, and cytotoxic T cells remains challenging. To overcome this, we loaded the engineered cytokine Neoleukin-2/15 (Neo2/15) on our recently established artificial antigen-presenting scaffolds (Ag-scaffolds) to expand antigen-specific T cells. Neo2/15 selectively binds to IL-2Rβ/γ receptors, enhancing CD8 + T cell proliferation while limiting regulatory T cell expansion. Our study assessed the efficacy of Neo2/15-loaded Ag-scaffolds (Ag-Neo2/15 scaffolds) in expanding antigen-specific T cells from peripheral blood mononuclear cells (PBMCs) of healthy donors. We optimized Ag-scaffold configurations by varying the number of Neo2/15 molecules loaded on Ag-scaffolds and evaluated their impact on T-cell expansion and functionality. We showed that Ag-Neo2/15 scaffolds promoted significant T-cell expansion, with a comparable frequency of antigen-specific CD8 + T cells compared to IL-2/IL-21-loaded Ag-scaffolds (Ag-IL2/21 scaffolds). The CD8 + T cells expanded with Ag-Neo2/15 scaffolds exhibited potent TNFα and IFNγ production and expressed high levels of α4β7 integrin, a homing molecule which is important for directing T cells to specific tissues, potentially enhancing their therapeutic potential. T cells expanded with Ag-Neo2/15 scaffolds had superior and durable cytotoxicity against tumor target cells compared to T cells expanded with Ag-IL2/21 scaffolds. These findings were further supported by our single-cell analysis revealing that T cells expanded with Ag-Neo2/15 scaffolds had higher cytotoxic scores and lower dysfunctionality scores compared to T cells expanded with Ag-IL2/21 scaffolds. The single-cell analysis also indicated increased expression of genes linked to cell division and enhanced proliferative capacity in Ag-Neo2/15 expanded T cells. Furthermore, TCR clonality analysis demonstrated that Ag-Neo2/15 scaffolds promoted the expansion of functionally superior T-cell clones. The top clones of CD8 + T cells expanded with Ag-Neo2/15 scaffolds exhibited a favorable phenotype, essential for effective antigen recognition and sustained T-cell mediated cytotoxicity. Our findings suggest that Ag-Neo2/15 scaffolds represent an advancement in ACT by producing high-quality, functional antigen-specific T cells. This method has the potential to improve clinical outcomes in cancer therapy by generating large numbers of highly functional T cells, thereby optimizing the balance between cytotoxicity and proliferation capacity with less exhausted T-cells in expansion protocols.
-
Immunology and Microbiology
In Journal for Immunotherapy of Cancer on 9 December 2024 by Fuchs, K. J., Göransson, M., et al.
Allogeneic stem cell transplantation (alloSCT) provides a curative treatment option for hematological malignancies. After HLA-matched alloSCT, donor-derived T cells recognize minor histocompatibility antigens (MiHAs), which are polymorphic peptides presented by HLA on patient cells. MiHAs are absent on donor cells due to genetic differences between patient and donor. T cells targeting broadly expressed MiHAs induce graft-versus-leukemia (GvL) reactivity as well as graft-versus-host disease (GvHD), while T cells for MiHAs with restricted or preferential expression on hematopoietic or non-hematopoietic cells may skew responses toward GvL or GvHD, respectively. Besides tissue expression, overall strength of GvL and GvHD is also determined by T-cell frequencies against MiHAs.Here, we explored the use of DNA barcode-labeled peptide-MHC multimers to detect and monitor antigen-specific T cells for the recently expanded repertoire of HLA-I-restricted MiHAs. In 16 patients who experienced an immune response after donor lymphocyte infusion, variable T-cell frequencies up to 30.5% of CD8+ T cells were measured for 49 MiHAs. High T-cell frequencies above 1% were measured in 12 patients for 19 MiHAs, with the majority directed against mismatched MiHAs, typically 6-8 weeks after donor lymphocyte infusion and at the onset of GvHD. The 12 patients included 9 of 10 patients with severe GvHD, 2 of 3 patients with limited GvHD and 1 of 3 patients without GvHD.In conclusion, we demonstrated that barcoded peptide-MHC multimers reliably detect and allow monitoring for MiHA-specific T cells during treatment to investigate the kinetics of immune responses and their impact on development of GvL and GvHD after HLA-matched alloSCT.
© Author(s) (or their employer(s)) 2024. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
-
Homo sapiens (Human)
-
Genetics
-
Immunology and Microbiology
-
Stem Cells and Developmental Biology
In Journal of Experimental & Clinical Cancer Research : CR on 20 March 2024 by Cavalluzzo, B., Viuff, M. C., et al.
We have recently shown extensive sequence and conformational homology between tumor-associated antigens (TAAs) and antigens derived from microorganisms (MoAs). The present study aimed to assess the breadth of T-cell recognition specific to MoAs and the corresponding TAAs in healthy subjects (HS) and patients with cancer (CP).
A library of > 100 peptide-MHC (pMHC) combinations was used to generate DNA-barcode labelled multimers. Homologous peptides were selected from the Cancer Antigenic Peptide Database, as well as Bacteroidetes/Firmicutes-derived peptides. They were incubated with CD8 + T cells from the peripheral blood of HLA-A*02:01 healthy individuals (n = 10) and cancer patients (n = 16). T cell recognition was identified using tetramer-staining analysis. Cytotoxicity assay was performed using as target cells TAP-deficient T2 cells loaded with MoA or the paired TuA.
A total of 66 unique pMHC recognized by CD8+ T cells across all groups were identified. Of these, 21 epitopes from microbiota were identified as novel immunological targets. Reactivity against selected TAAs was observed for both HS and CP. pMHC tetramer staining confirmed CD8+ T cell populations cross-reacting with CTA SSX2 and paired microbiota epitopes. Moreover, PBMCs activated with the MoA where shown to release IFNγ as well as to exert cytotoxic activity against cells presenting the paired TuA.
Several predicted microbiota-derived MoAs are recognized by T cells in HS and CP. Reactivity against TAAs was observed also in HS, primed by the homologous bacterial antigens. CD8+ T cells cross-reacting with MAGE-A1 and paired microbiota epitopes were identified in three subjects. Therefore, the microbiota can elicit an extensive repertoire of natural memory T cells to TAAs, possibly able to control tumor growth ("natural anti-cancer vaccination"). In addition, non-self MoAs can be included in preventive/therapeutic off-the-shelf cancer vaccines with more potent anti-tumor efficacy than those based on TAAs.
© 2024. The Author(s).
-
Homo sapiens (Human)
-
Cancer Research
-
Immunology and Microbiology
Preprint on BioRxiv : the Preprint Server for Biology on 17 March 2024 by Moss, K. H., Hansen, U. K., et al.
Background Immune checkpoint blockade (ICB) has been approved as first-line or second-line therapies for an expanding list of malignancies. T cells recognizing mutation-derived neoantigens are hypothesized to play a major role in tumor elimination. However, the dynamics and characteristics of such neoantigen-reactive T cells (NARTs) in the context of ICB are still limitedly understood. Methods To explore this, tumor biopsies and peripheral blood were obtained pre- and post-treatment from 20 patients with solid metastatic tumors, in a Phase I basket trial. From whole-exome sequencing and RNA-seq data, patient-specific libraries of neopeptides were predicted and screened with DNA barcode-labeled MHC multimers for CD8 + T cell reactivity, in conjunction with the evaluation of T cell phenotype. Results We were able to detect NARTs in the peripheral blood and tumor biopsies for the majority of the patients; however, we did not observe any significant difference between the disease control and progressive disease patient groups, in terms of the breadth and magnitude of the detected NARTs. We also observed that the hydrophobicity of the peptide played a role in defining neopeptides resulting in NARTs response. A trend towards a treatment-induced phenotype signature was observed in the NARTs post-treatment, with the appearance of Ki67 + CD27 + PD-1 + subsets in the PBMCs and CD39 + Ki67 + TCF-1 + subsets in the TILs. Finally, the estimation of T cells from RNAseq was increasing post versus pre-treatment for disease control patients. Conclusion Our data demonstrates the possibility of monitoring the characteristics of NARTs from tumor biopsies and peripheral blood, and that such characteristics could potentially be incorporated with other immune predictors to understand further the complexity governing clinical success for ICB therapy.
-
Homo sapiens (Human)
-
Cancer Research
-
Immunology and Microbiology
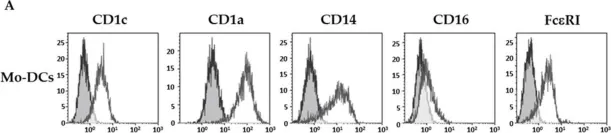
In Cells on 2 July 2021 by Coutant, F., Pin, J. J., et al.
Fig.1.A

-
FC/FACS
-
Collected and cropped from Cells by CiteAb, provided under a CC-BY license
Image 1 of 2
In PLoS One on 21 November 2018 by Alshareeda, A., Rakha, E., et al.
Fig.1.B

-
FC/FACS
-
Homo sapiens (Human)
Collected and cropped from PLoS One by CiteAb, provided under a CC-BY license
Image 1 of 2