Acute myeloid leukemia (AML) remains challenging to cure. In addition to mutations that alter cell functioning, biophysical properties are modulated by external cues. In particular, membrane proteins that interact with the bone marrow niche can induce cellular changes. Here, we develop an atomic force microscopy (AFM) approach to measure non-adherent AML cell mechanical properties. The Young's modulus of the AML cell line, THP-1, increased in response to retronectin, whereas knock-out of the adhesion protein ITGB1 resulted in no response to retronectin. Confocal microscopy revealed different actin cytoskeleton morphologies for wild-type and ITGB1 knock-out cells exposed to retronectin. These results indicate that ITGB1 mediates stimuli-induced cellular mechanoresponses through cytoskeletal changes. We next used AFM to investigate the elastic properties of primary AML cells and found that more committed cells had lower Young's moduli than immature AMLs. Overall, this provides a platform for investigating the molecular mechanisms involved in leukemic cell mechanoresponse.
© 2025 The Author(s).
Product Citations: 52
Elastic properties of leukemic cells linked to maturation stage and integrin activation.
In IScience on 18 April 2025 by Richards, C. J., Wierenga, A. T. J., et al.
In Experimental Physiology on 1 February 2025 by Ross, M., Aldred, S., et al.
CD34+ progenitor cells with angiogenic capabilities traffic into blood during exercise and extravasate afterwards but the magnitude of this response varies between people. We examined whether exercise-induced progenitor cell trafficking is influenced by cardiorespiratory fitness (maximum oxygen uptake; V ̇ O 2 max ${{\dot{V}}_{{{{\mathrm{O}}}_2}{\mathrm{max}}}}$ ). Ten males (age: 23 ± 3 years; V ̇ O 2 max ${{\dot{V}}_{{{{\mathrm{O}}}_2}{\mathrm{max}}}}$ : 61.88 ± 4.68 mL kg min-1) undertook 1 h of treadmill running at 80% of V ̇ O 2 max ${{\dot{V}}_{{{{\mathrm{O}}}_2}{\mathrm{max}}}}$ . Blood samples were collected before exercise (Pre), in the final minute of exercise (0 h) and afterwards at 0.25, 1 and 24 h. Pan-progenitor cells (CD34+, CD34+CD45dim) and putative endothelial progenitor cells (CD34+CD133+, CD34+VEGFR2+, CD34+CD45dimVEGFR2+) were quantified using flow cytometry. Progenitor subpopulations (except for CD34+CD45dimVEGFR2+) increased at 0 h (P < 0.05) and returned to pre-exercise levels by 1 h. V ̇ O 2 max ${{\dot{V}}_{{{{\mathrm{O}}}_2}{\mathrm{max}}}}$ was positively associated with the exercise-induced progenitor cell response and there were statistically significant time × V ̇ O 2 max ${{\dot{V}}_{{{{\mathrm{O}}}_2}{\mathrm{max}}}}$ interactions for CD34+, CD34+CD45dim and CD34+CD133+ subpopulations but not VEGFR2-expressing progenitor cells. There were statistically significant correlations between V ̇ O 2 max ${{\dot{V}}_{{{{\mathrm{O}}}_2}{\mathrm{max}}}}$ and ingress (r > 0.70, P < 0.025) and egress (r > -0.77, P < 0.009) of progenitor cell subsets (CD34+, CD34+CD45dim, CD34+CD133+), showing that cardiorespiratory fitness influences the magnitude of progenitor cell mobilisation into the blood and subsequent extravasation. These data may provide a link between high levels of cardiorespiratory fitness and vascular health.
© 2024 The Author(s). Experimental Physiology published by John Wiley & Sons Ltd on behalf of The Physiological Society.
-
Endocrinology and Physiology
In Molecular Therapy. Methods Clinical Development on 12 September 2024 by Crippa, S., Alberti, G., et al.
Mucopolysaccharidosis type IVB (MPSIVB) is a lysosomal storage disorder caused by β-galactosidase (β-GAL) deficiency characterized by severe skeletal and neurological alterations without approved treatments. To develop hematopoietic stem progenitor cell (HSPC) gene therapy (GT) for MPSIVB, we designed lentiviral vectors (LVs) encoding human β-GAL to achieve supraphysiological release of the therapeutic enzyme in human HSPCs and metabolic correction of diseased cells. Transduced HSPCs displayed proper colony formation, proliferation, and differentiation capacity, but their progeny failed to release the enzyme at supraphysiological levels. Therefore, we tested alternative LVs to overexpress an enhanced β-GAL deriving from murine (LV-enhGLB1) and human selectively mutated GLB1 sequences (LV-mutGLB1). Only human HSPCs transduced with LV-enhGLB1 overexpressed β-GAL in vitro and in vivo without evidence of overexpression-related toxicity. Their hematopoietic progeny efficiently released β-GAL, allowing the cross-correction of defective cells, including skeletal cells. We found that the low levels of human GLB1 mRNA in human hematopoietic cells and the improved stability of the enhanced β-GAL contribute to the increased efficacy of LV-enhGLB1. Importantly, the enhanced β-GAL enzyme showed physiological lysosomal trafficking in human cells and was not associated with increased immunogenicity in vitro. These results support the use of LV-enhGLB1 for further HSPC-GT development and future clinical translation to treat MPSIVB multisystem disease.
© 2024 The Authors.
In Cell Reports Medicine on 21 May 2024 by Adnan Awad, S., Dufva, O., et al.
BCR::ABL1-independent pathways contribute to primary resistance to tyrosine kinase inhibitor (TKI) treatment in chronic myeloid leukemia (CML) and play a role in leukemic stem cell persistence. Here, we perform ex vivo drug screening of CML CD34+ leukemic stem/progenitor cells using 100 single drugs and TKI-drug combinations and identify sensitivities to Wee1, MDM2, and BCL2 inhibitors. These agents effectively inhibit primitive CD34+CD38- CML cells and demonstrate potent synergies when combined with TKIs. Flow-cytometry-based drug screening identifies mepacrine to induce differentiation of CD34+CD38- cells. We employ genome-wide CRISPR-Cas9 screening for six drugs, and mediator complex, apoptosis, and erythroid-lineage-related genes are identified as key resistance hits for TKIs, whereas the Wee1 inhibitor AZD1775 and mepacrine exhibit distinct resistance profiles. KCTD5, a consistent TKI-resistance-conferring gene, is found to mediate TKI-induced BCR::ABL1 ubiquitination. In summary, we delineate potential mechanisms for primary TKI resistance and non-BCR::ABL1-targeting drugs, offering insights for optimizing CML treatment.
Copyright © 2024 The Authors. Published by Elsevier Inc. All rights reserved.
-
Cancer Research
In Frontiers in Immunology on 12 June 2023 by Ait Djebbara, S., Mcheik, S., et al.
We previously identified the recombinant (r) macrophage (M) infectivity (I) potentiator (P) of the protozoan parasite Trypanosoma cruzi (Tc) (rTcMIP) as an immuno-stimulatory protein that induces the release of IFN-γ, CCL2 and CCL3 by human cord blood cells. These cytokines and chemokines are important to direct a type 1 adaptive immune response. rTcMIP also increased the Ab response and favored the production of the Th1-related isotype IgG2a in mouse models of neonatal vaccination, indicating that rTcMIP could be used as a vaccine adjuvant to enhance T and B cell responses. In the present study, we used cord and adult blood cells, and isolated NK cells and human monocytes to investigate the pathways and to decipher the mechanism of action of the recombinant rTcMIP. We found that rTcMIP engaged TLR1/2 and TLR4 independently of CD14 and activated the MyD88, but not the TRIF, pathway to induce IFN-γ production by IL-15-primed NK cells, and TNF-α secretion by monocytes and myeloid dendritic cells. Our results also indicated that TNF-α boosted IFN-γ expression. Though cord blood cells displayed lower responses than adult cells, our results allow to consider rTcMIP as a potential pro-type 1 adjuvant that might be associated to vaccines administered in early life or later.
Copyright © 2023 Ait Djebbara, Mcheik, Percier, Segueni, Poncelet and Truyens.
-
FC/FACS
-
Cardiovascular biology
-
Immunology and Microbiology
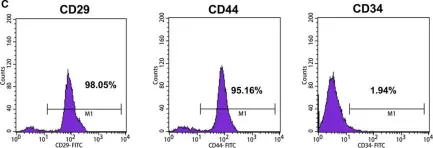
In J Cell Mol Med on 1 October 2018 by Sun, J., Zhao, F., et al.
Fig.1.C

-
FC/FACS
-
Rattus norvegicus (Rat)
Collected and cropped from J Cell Mol Med by CiteAb, provided under a CC-BY license
Image 1 of 2
In PLoS One on 31 July 2012 by Ferro, F., Spelat, R., et al.
Fig.4.A

-
FC/FACS
-
Homo sapiens (Human)
Collected and cropped from PLoS One by CiteAb, provided under a CC-BY license
Image 1 of 2