It is well known that multiple myeloma (MM) cells are highly dependent on the bone marrow (BM) microenvironment. However, the complex interactions and signaling pathways between MM cells and BM stromal cells remain inadequately defined. In this study, we employed an in vitro coculture model to investigate these interactions. We found that coculturing MM cells with BM-derived HS5 stromal cells stimulated the secretion of hyaluronic acid (HA) and interleukin-6 (IL-6), and significantly increased the expression of CD44 and F-actin stress fibers polymerization in MM cells. Among the three hyaluronan synthase (HAS) isoforms, HAS3 mRNA expression was most significantly elevated in MM cells following coculture with HS5. This coculture also resulted in upregulation of HAS3 and IL-6 mRNA in MM cells. Notably, MM cells in direct contact with HS5 cells exhibited higher proliferative capacity compared to those not in contact with the stromal cells. Additionally, coculturing MM cells with HS5 led to the formation of membrane protrusions in MM cells, with CD44 enrichment observed at these polarized regions. Further analysis revealed that Rac1 co-localizes with CD44 on MM cells within the coculture system, suggesting that Rac1 signaling plays a critical role in CD44-mediated cytoskeletal rearrangements. Importantly, silencing CD44 expression in MM cells reduced F-actin polymerization, as well as impaired MM cell migration and adhesion to HS5. Our findings highlight the involvement of the HA/CD44/F-actin pathway in MM-BM migration and adhesion, suggesting that CD44 may serve as a novel therapeutic target to disrupt the MM-BM microenvironment.
© 2025. The Author(s).
Product Citations: 7
In Clinical and Experimental Medicine on 7 June 2025 by Wang, Z., Guo, Y., et al.
-
Cell Biology
In Frontiers in Immunology on 3 January 2023 by Graciliano, N. G., Tenório, M. C. S., et al.
Limited data are available regarding the differences between immunological, biochemical, and cellular contents of human colostrum following maternal infection during pregnancy with coronavirus 2 disease (COVID-19).
To investigate whether maternal COVID-19 infection may affect immunological, biochemical, and cellular contents of human colostrum.
Using a case-control study design, we collected colostrum from 14 lactating women with a previous diagnosis of COVID-19 during pregnancy and 12 without a clear diagnosis during September 2020 to May 2021. Colostrum samples were analysed for some enzymes and non-enzymatic oxidative stress markers (SOD, CAT, GPx, MDA, GSH, GSSG, H2O2, MPO) and for IL-1β, IL-6, tumour necrosis factor (TNF)-α, protein induced by interferon gamma (IP)-10, IL-8, IFN-λ1, IL12p70, IFN-α2, IFN-λ2/3, granulocyte macrophage colony stimulating factor (GM-CSF), IFN-β, IL-10 and IFN-γ, along with IgA and IgG for the SARS-CoV-2 S protein. We perform immunophenotyping to assess the frequency of different cell types in the colostrum.
Colostrum from the COVID-19 symptomatic group in pregnancy contained reduced levels of H2O2, IFN-α2, and GM-CSF. This group had higher levels of GSH, and both NK cell subtypes CD3-CD56brightCD16-CD27+IFN-γ+ and CD3-CD56dimCD16+CD27- were also increased.
The present results reinforce the protective role of colostrum even in the case of mild SARS-Cov-2 infection, in addition to demonstrating how adaptive the composition of colostrum is after infections. It also supports the recommendation to encourage lactating women to continue breastfeeding after COVID-19 illness.
Copyright © 2022 Graciliano, Tenório, Fragoso, Moura, Botelho, Tanabe, Borbely, Borbely, Oliveira and Goulart.
-
COVID-19
-
Endocrinology and Physiology
-
Immunology and Microbiology
Post SARS-CoV-2 cell mediated Immune profiles; Case studies
Preprint on MedRxiv : the Preprint Server for Health Sciences on 5 July 2022 by Singh, R., Ravichandiran, V., et al.
Cell-mediated immunity (CMI), which includes T-cells (both T helper and cytotoxic), is critical for effective antiviral defenses against coronavirus disease-2019 (COVID-19). To better understand the immunological characteristics of CD markers on T-cells in post-COVID-19 patients, we investigated the expression of differential CD markers in the patient groups in this study. Flow cytometry was used to quantify total lymphocyte count and assess the levels of expression of CD markers in the samples. The percentage of Lymphocytes decreased significantly in the post-SARS-COV-2 patients in comparison to normal subjects, which is usually happening in any viral infection. In contrast to that, expression of CD8 was increased in the patient group having long SARS-COV-2 infection with comorbid complications with respect to the normal individuals and long SARS-COV-2 infection without comorbid complications. This data revealed that the cellular immunological responses corroborated with an earlier report of COVID-19 infection were mediated by CD8 upregulation and cytotoxic T lymphocyte hyperactivation.
-
COVID-19
-
Immunology and Microbiology
A bispecific CAR-T cell therapy targeting BCMA and CD38 in relapsed or refractory multiple myeloma.
In Journal of Hematology & Oncology on 9 October 2021 by Mei, H., Li, C., et al.
BCMA-specific chimeric antigen receptor-T cells (CAR-Ts) have exhibited remarkable efficacy in refractory or relapsed multiple myeloma (RRMM); however, primary resistance and relapse exist with single-target immunotherapy. Bispecific CARs are proposed to mitigate these limitations.
We constructed a humanized bispecific BM38 CAR targeting BCMA and CD38 and tested the antimyeloma activity of BM38 CAR-Ts in vitro and in vivo. Twenty-three patients with RRMM received infusions of BM38 CAR-Ts in a phase I trial.
BM38 CAR-Ts showed stronger in vitro cytotoxicity to heterogeneous MM cells than did T cells expressing an individual BCMA or CD38 CAR. BM38 CAR-Ts also exhibited potent antimyeloma activity in xenograft mouse models. In the phase I trial, cytokine release syndrome occurred in 20 patients (87%) and was mostly grade 1-2 (65%). Neurotoxicity was not observed. Hematologic toxicities were common, including neutropenia in 96% of the patients, leukopenia in 87%, anemia in 43% and thrombocytopenia in 61%. At a median follow-up of 9.0 months (range 0.5 to 18.5), 20 patients (87%) attained a clinical response and minimal residual disease-negativity (≤ 10-4 nucleated cells), with 12 (52%) achieving a stringent complete response. Extramedullary plasmacytoma was eliminated completely in 56% and partially in 33% and of 9 patients. The median progression-free survival was 17.2 months. Two relapsed patients maintained BCMA and CD38 expression on MM cells. Notably, BM38 CAR-Ts cells were detectable in 77.8% of evaluable patients at 9 months and 62.2% at 12 months.
Bispecific BM38 CAR-Ts were feasible, safe and significantly effective in patient with RRMM.
Chictr.org.cn ChiCTR1800018143.
© 2021. The Author(s).
-
Immunology and Microbiology
Isolation of Human CD138(+) Microparticles from the Plasma of Patients with Multiple Myeloma.
In Neoplasia (New York, N.Y.) on 1 January 2016 by Krishnan, S. R., Luk, F., et al.
The confinement of multiple myeloma (MM) to the bone marrow microenvironment requires an invasive bone marrow biopsy to monitor the malignant compartment. The existing clinical tools used to determine treatment response and tumor relapse are limited in sensitivity mainly because they indirectly measure tumor burden inside the bone marrow and fail to capture the patchy, multisite tumor infiltrates associated with MM. Microparticles (MPs) are 0.1- to 1.0-μm membrane vesicles, which contain the cellular content of their originating cell. MPs are functional mediators and convey prothrombotic, promalignant, proresistance, and proinflammatory messages, establishing intercellular cross talk and bypassing the need for direct cell-cell contact in many pathologies. In this study, we analyzed plasma cell-derived MPs (CD138(+)) from deidentified MM patients (n = 64) and normal subjects (n = 18) using flow cytometry. The morphology and size of the MPs were further analyzed using scanning electron microscopy. Our study shows the proof of a systemic signature of MPs in MM patients. We observed that the levels of MPs were significantly elevated in MM corresponding to the tumor burden. We provide the first evidence for the presence of MPs in the peripheral blood of MM patients with potential applications in personalized MM clinical monitoring.
Copyright © 2015 Institut National de la Santé Et de la Recherche Médicale. Published by Elsevier Inc. All rights reserved.
-
Homo sapiens (Human)
-
Cancer Research
In Oncotarget on 13 October 2015 by Canella, A., Cordero Nieves, H., et al.
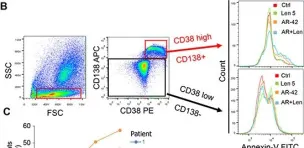
Fig.5.B
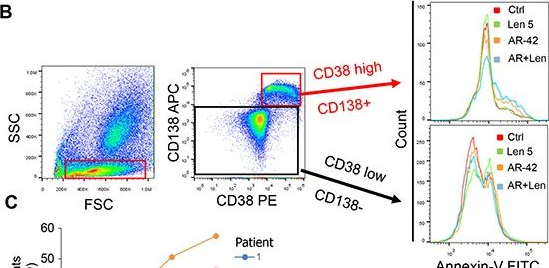
-
FC/FACS
-
Collected and cropped from Oncotarget by CiteAb, provided under a CC-BY license
Image 1 of 1