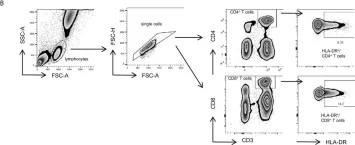
Patients with acute myeloid leukemia (AML) often carry the same gene mutations. Neoantigens encoded by these mutations are attractive targets for immunotherapy.
We searched for public human leukocyte antigen (HLA) class II-restricted neoantigens on AML using an in vitro T cell stimulation method. Peptides from 26 recurrent genetic aberrations were assessed for predicted HLA class II binding, and 24 long neopeptides encoded by 10 recurrent mutations were synthesized. Naive CD4 T cells from healthy individuals were cocultured with autologous dendritic cells pulsed with neopeptides.
Multiple CD4 T cell clones were isolated that recognized neopeptides encoded by 5 different genetic aberrations. Two of these peptides, one from the well-known DNMT3A-R882H hotspot mutation and one from a long alternative reading frame created by frameshift mutations in RUNX1, were recognized by CD4 T cell clones after endogenous processing and presentation on cell lines transduced or CRISPR-Cas9-edited with the mutation of interest. The T cell clone for DNMT3A-R882H was also activated upon stimulation with primary AML samples from HLA-DQB1*06:02 or -DQB1*06:03 positive patients with the mutation.
We here identified a public HLA class II-restricted neoantigen encoded by a driver mutation occurring in 10% of patients with AML that could become an important target for immunotherapy to treat patients with DNMT3A-R882H-mutated AML.
Copyright © 2025 van der Lee, Argiro, Laan, Honders, de Jong, Struckman, Falkenburg and Griffioen.
Product Citations: 64
Mutated DNMT3A creates a public HLADQ- binding neoantigen on acute myeloid leukemia.
In Frontiers in Immunology on 28 March 2025 by van der Lee, D. I., Argiro, E. M., et al.
-
FC/FACS
-
Homo sapiens (Human)
-
Cancer Research
-
Immunology and Microbiology
In Frontiers in Oncology on 7 February 2023 by Schoeppe, R., Babl, N., et al.
Glutamine deficiency is a well-known feature of the tumor environment. Here we analyzed the impact of glutamine deprivation on human myeloid cell survival and function.
Different types of myeloid cells were cultured in the absence or presence of glutamine and/or with L-methionine-S-sulfoximine (MSO), an irreversible glutamine synthetase (GS) inhibitor. GS expression was analyzed on mRNA and protein level. GS activity and the conversion of glutamate to glutamine by myeloid cells was followed by 13C tracing analyses.
The absence of extracellular glutamine only slightly affected postmitotic human monocyte to dendritic cell (DC) differentiation, function and survival. Similar results were obtained for monocyte-derived macrophages. In contrast, proliferation of the monocytic leukemia cell line THP-1 was significantly suppressed. While macrophages exhibited high constitutive GS expression, glutamine deprivation induced GS in DC and THP-1. Accordingly, proliferation of THP-1 was rescued by addition of the GS substrate glutamate and 13C tracing analyses revealed conversion of glutamate to glutamine. Supplementation with the GS inhibitor MSO reduced the survival of DC and macrophages and counteracted the proliferation rescue of THP-1 by glutamate.
Our results show that GS supports myeloid cell survival in a glutamine poor environment. Notably, in addition to suppressing proliferation and survival of tumor cells, the blockade of GS also targets immune cells such as DCs and macrophages.
Copyright © 2023 Schoeppe, Babl, Decking, Schönhammer, Siegmund, Bruss, Dettmer, Oefner, Frick, Weigert, Jantsch, Herr, Rehli, Renner and Kreutz.
-
FC/FACS
-
Immunology and Microbiology
In Cells on 8 July 2022 by Vives, J., Hernandez, J., et al.
(1) Background: the use of Mesenchymal Stromal Cells (MSC) in emerging therapies for spinal cord injury (SCI) hold the potential to improve functional recovery. However, the development of cell-based medicines is challenging and preclinical studies addressing quality, safety and efficacy must be conducted prior to clinical testing; (2) Methods: herein we present (i) the characterization of the quality attributes of MSC from the Wharton's jelly (WJ) of the umbilical cord, (ii) safety of intrathecal infusion in a 3-month subchronic toxicity assessment study, and (iii) efficacy in a rat SCI model by controlled impaction (100 kdynes) after single (day 7 post-injury) and repeated dose of 1 × 106 MSC,WJ (days 7 and 14 post-injury) with 70-day monitoring by electrophysiological testing, motor function assessment and histology evaluation; (3) Results: no toxicity associated to MSC,WJ infusion was observed. Regarding efficacy, recovery of locomotion was promoted at early time points. Persistence of MSC,WJ was detected early after administration (day 2 post-injection) but not at days 14 and 63 post-injection. (4) Conclusions: the safety profile and signs of efficacy substantiate the suitability of the presented data for inclusion in the Investigational Medicinal Product Dossier for further consideration by the competent Regulatory Authority to proceed with clinical trials.
-
FC/FACS
-
Cell Biology
-
Neuroscience
In Heliyon on 1 April 2022 by Townsend, L., Dyer, A. H., et al.
SARS-CoV-2 infection causes a wide spectrum of disease severity. Identifying the immunological characteristics of severe disease and the risk factors for their development are important in the management of COVID-19. This study aimed to identify and rank clinical and immunological features associated with progression to severe COVID-19 in order to investigate an immunological signature of severe disease. One hundred and eight patients with positive SARS-CoV-2 PCR were recruited. Routine clinical and laboratory markers were measured, as well as myeloid and lymphoid whole-blood immunophenotyping and measurement of the pro-inflammatory cytokines IL-6 and soluble CD25. All analysis was carried out in a routine hospital diagnostic laboratory. Univariate analysis demonstrated that severe disease was most strongly associated with elevated CRP and IL-6, loss of DLA-DR expression on monocytes and CD10 expression on neutrophils. Unbiased machine learning demonstrated that these four features were strongly associated with severe disease, with an average prediction score for severe disease of 0.925. These results demonstrate that these four markers could be used to identify patients developing severe COVID-19 and allow timely delivery of therapeutics.
© 2022 The Author(s).
-
COVID-19
-
Immunology and Microbiology
In Nature Methods on 1 January 2022 by Albanese, M., Ruhle, A., et al.
CD4+ T cells are central mediators of adaptive and innate immune responses and constitute a major reservoir for human immunodeficiency virus (HIV) in vivo. Detailed investigations of resting human CD4+ T cells have been precluded by the absence of efficient approaches for genetic manipulation limiting our understanding of HIV replication and restricting efforts to find a cure. Here we report a method for rapid, efficient, activation-neutral gene editing of resting, polyclonal human CD4+ T cells using optimized cell cultivation and nucleofection conditions of Cas9-guide RNA ribonucleoprotein complexes. Up to six genes, including HIV dependency and restriction factors, were knocked out individually or simultaneously and functionally characterized. Moreover, we demonstrate the knock in of double-stranded DNA donor templates into different endogenous loci, enabling the study of the physiological interplay of cellular and viral components at single-cell resolution. Together, this technique allows improved molecular and functional characterizations of HIV biology and general immune functions in resting CD4+ T cells.
© 2021. The Author(s).
-
Immunology and Microbiology
In Front Immunol on 3 August 2021 by Schäfer, A. L., Eichhorst, A., et al.
Fig.5.B

-
FC/FACS
-
Collected and cropped from Front Immunol by CiteAb, provided under a CC-BY license
Image 1 of 1