Acute leukemia relapsing after chemotherapy plus allogeneic hematopoietic stem cell transplantation can be treated with donor-derived T cells, but this is hampered by the need for donor/recipient MHC-matching and often results in graft-versus-host disease, prompting the search for new donor-unrestricted strategies targeting malignant cells. Leukemia blasts express CD1c antigen-presenting molecules, which are identical in all individuals and expressed only by mature leukocytes, and are recognized by T cell clones specific for the CD1c-restricted leukemia-associated methyl-lysophosphatidic acid (mLPA) lipid antigen. Here, we show that human T cells engineered to express an mLPA-specific TCR, target diverse CD1c-expressing leukemia blasts in vitro and significantly delay the progression of three models of leukemia xenograft in NSG mice, an effect that is boosted by mLPA-cellular immunization. These results highlight a strategy to redirect T cells against leukemia via transfer of a lipid-specific TCR that could be used across MHC barriers with reduced risk of graft-versus-host disease.
© 2021. The Author(s).
Product Citations: 11
In Nature Communications on 11 August 2021 by Consonni, M., Garavaglia, C., et al.
-
Cancer Research
-
Immunology and Microbiology
In Head and Neck Pathology on 1 September 2020 by Norouzian, M., Mehdipour, F., et al.
Research on the role of B cells in the development and modulation of antitumor immunity has increased in recent years; however, knowledge about B cell phenotype and function in tumor draining lymph nodes (TDLNs) is still incomplete. This study aimed to investigate changes in the phenotypic profile of B cells in TDLNs of head and neck squamous cell carcinoma (HNSCC) during disease progression. Mononuclear cells were isolated from TDLNs and stained with antibodies for CD19 and other B cell-related markers and analyzed by flow cytometry. CD19+ B cells comprised 38.6 ± 8.9% of lymphocytes in TDLNs of HNSCC. Comparison of metastatic and non-metastatic LNs disclosed no significant differences in the frequencies of B cell subsets including antigen-experienced, naïve, switched, unswitched, atypical memory, marginal zone-like B cells, and B cells with regulatory phenotypes. The percentage of atypical memory (CD27-IgM-IgD-) B cells was significantly higher in patients with tongue SCC with no involved LNs (p = 0.033) and correlated inversely with the number of involved LNs. The frequency of CD24hiCD38hi B cells was significantly higher in non-metastatic LNs of patients with grade I compared to grade II (p = 0.016), and the percentage of CD5+ B cells decreased as tumors progressed from stage III to IV (p = 0.008). Our data show that in TDLNs of HNSCC, the frequency of B cells with atypical memory and regulatory phenotypes was significantly associated with good prognostic factors; however, their function remains to be investigated.
-
Cancer Research
-
Immunology and Microbiology
-
Pathology
In Nature Communications on 10 April 2018 by Joas, S., Parrish, E. H., et al.
HIV-1 causes chronic inflammation and AIDS in humans, whereas related simian immunodeficiency viruses (SIVs) replicate efficiently in their natural hosts without causing disease. It is currently unknown to what extent virus-specific properties are responsible for these different clinical outcomes. Here, we incorporate two putative HIV-1 virulence determinants, i.e., a Vpu protein that antagonizes tetherin and blocks NF-κB activation and a Nef protein that fails to suppress T cell activation via downmodulation of CD3, into a non-pathogenic SIVagm strain and test their impact on viral replication and pathogenicity in African green monkeys. Despite sustained high-level viremia over more than 4 years, moderately increased immune activation and transcriptional signatures of inflammation, the HIV-1-like SIVagm does not cause immunodeficiency or any other disease. These data indicate that species-specific host factors rather than intrinsic viral virulence factors determine the pathogenicity of primate lentiviruses.
-
FC/FACS
-
Homo sapiens (Human)
-
Immunology and Microbiology
In Oncology Letters on 1 February 2018 by Bojarska-Junak, A., Waldowska, M., et al.
Malignant B cells in chronic lymphocytic leukemia serve an essential role in the whole immune response, so their interactions with other immune cells are more complex than observed in solid tumors. The latest study results indicate that the immune dysregulation in chronic lymphocytic leukemia (CLL) also affects a small population of invariant natural killer T cells (iNKT). Using peripheral blood iNKT cells obtained from patients with CLL, the objective of the present study was to assess the intracellular expression of typical cytokines involved in the Th1 (IFN-γ) and Th2 (IL-4) response pathways following stimulation with the iNKT-specific ligand α-galactosylceramide. iNKT cells from patients with CLL exhibited upregulated IL-4 and IFN-γ expression in comparison to those from HVs. No significant association between the ability of iNKT cells to produce IL-4 or IFN-γ and the expression of CD1d on leukemic B lymphocytes or monocytes was identified. However, the function of iNKT cells was compromised in patients with CLL by a strong Th2 bias (high IL-4 and low IFN-γ expression). The ratio of iNKT+IFN-γ+:iNKT+IL-4+ was significantly decreased in the CLL group when compared with HVs, and this decreased further as the disease progressed. This change may result in the promotion of leukemic B lymphocyte survival. Therefore, in the pathogenesis of CLL, Th2 bias may delay the antitumor response that relies on stimulation of the Th1 immune response.
-
FC/FACS
-
Homo sapiens (Human)
-
Cancer Research
Vpu-Mediated Counteraction of Tetherin Is a Major Determinant of HIV-1 Interferon Resistance.
In mBio on 16 August 2016 by Kmiec, D., Iyer, S. S., et al.
Human immunodeficiency virus type 1 (HIV-1) groups M, N, O, and P are the result of independent zoonotic transmissions of simian immunodeficiency viruses (SIVs) infecting great apes in Africa. Among these, only Vpu proteins of pandemic HIV-1 group M strains evolved potent activity against the restriction factor tetherin, which inhibits virus release from infected cells. Thus, effective Vpu-mediated tetherin antagonism may have been a prerequisite for the global spread of HIV-1. To determine whether this particular function enhances primary HIV-1 replication and interferon resistance, we introduced mutations into the vpu genes of HIV-1 group M and N strains to specifically disrupt their ability to antagonize tetherin, but not other Vpu functions, such as degradation of CD4, down-modulation of CD1d and NTB-A, and suppression of NF-κB activity. Lack of particular human-specific adaptations reduced the ability of HIV-1 group M Vpu proteins to enhance virus production and release from primary CD4(+) T cells at high levels of type I interferon (IFN) from about 5-fold to 2-fold. Interestingly, transmitted founder HIV-1 strains exhibited higher virion release capacity than chronic control HIV-1 strains irrespective of Vpu function, and group M viruses produced higher levels of cell-free virions than an N group HIV-1 strain. Thus, efficient virus release from infected cells seems to play an important role in the spread of HIV-1 in the human population and requires a fully functional Vpu protein that counteracts human tetherin.
Understanding which human-specific adaptations allowed HIV-1 to cause the AIDS pandemic is of great importance. One feature that distinguishes pandemic HIV-1 group M strains from nonpandemic or rare group O, N, and P viruses is the acquisition of mutations in the accessory Vpu protein that confer potent activity against human tetherin. Adaptation was required because human tetherin has a deletion that renders it resistant to the Nef protein used by the SIV precursor of HIV-1 to antagonize this antiviral factor. It has been suggested that these adaptations in Vpu were critical for the effective spread of HIV-1 M strains, but direct evidence has been lacking. Here, we show that these changes in Vpu significantly enhance virus replication and release in human CD4(+) T cells, particularly in the presence of IFN, thus supporting an important role in the spread of pandemic HIV-1.
Copyright © 2016 Kmiec et al.
-
FC/FACS
-
Homo sapiens (Human)
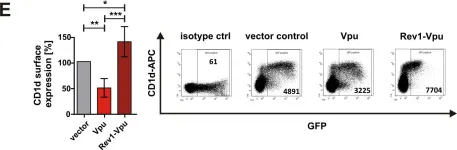
In PLoS One on 12 November 2015 by Langer, S. M., Hopfensperger, K., et al.
Fig.3.E

-
FC/FACS
-
Homo sapiens (Human)
Collected and cropped from PLoS One by CiteAb, provided under a CC-BY license
Image 1 of 1