Adipose tissue secretome plays a crucial role in the mechanisms of metabolic diseases. Weight loss has a favourable effect on the adipose tissue secretome and prevents the development of type 2 diabetes mellitus (T2DM) and its complications. The most effective methods of glycaemic control are bariatric surgery (BS) and pharmacotherapy. The aim of our study is to evaluate changes in adipose tissue secretome after BS and semaglutide injections.
17 patients with T2DM were examined before and 6 months after BS or semaglutide therapy. The examination protocol included anthropometry, clinical biochemistry, insulin resistance evaluation and collection of subcutaneous adipose tissue biopsies. Adipose derived stem cells (ADSC) were isolated from biopsies according to a standard enzymatic protocol and differentiated into white and beige adipocytes. Adipogenesis and thermogenesis were assessed by confocal microscopy. Secretome of adipocytes and cytokines plasma levels were analyzed using a MILLIPLEX panel.
Following BS and semaglutide therapy, a decline in BMI, total fat content, HbA1c, and fasting blood glucose was observed. Insulin sensitivity increased only 6 months after BS. Semaglutide therapy resulted in the elevation of angiogenic and proinflammatory cytokines in adipocyte secretory profile. After BS we also detected the increase in proinflammatory cytokines both in adipocyte secretome and in plasma levels. However, the adipocyte secretome subsequent to bariatric surgery (BS) exhibited a reduced proinflammatory response in comparison to that observed following semaglutide therapy.
The effect of semaglutide injections directly on adipose tissue can change the function of ADSC, making them more angiogenic and adipogenic. A decrease in BMI, HbA1c and insulin resistance is achieved to a significant extent only after BS. BS-induced T2DM remission is related to lower pro-inflammatory secretion from adipocytes as compared to semaglutide. The regulation of inflammation in adipocytes may serve as a potential mechanism underlying BS-induced T2DM remission.
© 2025. The Author(s).
Product Citations: 388
In BMC Endocrine Disorders on 17 July 2025 by Agareva, M., Michurina, S., et al.
-
Endocrinology and Physiology
Preprint on Research Square on 28 April 2025 by Rauch, A., Jamil, A., et al.
Abstract Stromal progenitor cells of bone marrow origin are non-hematopoietic cells that give rise to osteoblasts and adipocytes in the postnatal organism. Marrow stromal cells (MSCs) (also known as skeletal stem cells)are currently being employed in a large number of clinical trials for regenerative purposes post in vitro expansion. However, the clinical outcome has been variable, which might in part be due to the heterogeneity of the cells and the lack of a defined cell product with a molecular signature that favors tissue regeneration. In this study, we determined the cellular heterogeneity of primary stromal cultures and examined how inter-donor variation in subpopulation composition contributes to the differentiation potential of primary cultures. We profiled 136,014 stromal progenitors from 26 donors and identified 5 subpopulations that were linked to distinct bone-related pathways and genetic traits of bone mineral density and morphology. Abundancy of one cluster characterized by high expression of ITGA11 and genes related to matrix function, collagen organization, and elevated expression upon lineage commitment was positively correlated with osteoblastic differentiation capacity in vitro. In addition, ITGA11 protein expression in progenitor cells was a predictive marker for matrix mineralization in vitro and ectopic bone formation in vivo. Sorting stromal progenitors into ITGA11high and ITGA11low cells established cultures with high and low osteoblastic differentiation potential, respectively. Our findings corroborate the presence of extensive cellular heterogeneity among cultured human stromal cells, which strongly differs from donor to donor, and that ITGA11 can be employed as a marker for isolating cells with high bone-forming potential, a feature likely to benefit clinical trials of bone regeneration.
-
FC/FACS
In Scientific Reports on 22 February 2025 by Lee, J., Min, H. K., et al.
This study aimed to investigate the therapeutic effect of human nasal turbinate-derived stem cells (hNTSCs) on mice with rheumatoid arthritis (RA) and identify hNTSC gene signatures with therapeutic effects on RA. hNTSCs were obtained from 20 healthy controls (HCs) who had undergone nasal turbinate surgery. Collagen-induced arthritis (CIA) mice were used to investigate the therapeutic effects of hNTSCs. The engraftment and migration abilities of hNTSCs were evaluated. CD4+CD25- T cells were co-cultured with hNTSCs, and effector T cell proliferation was evaluated by flow cytometry. Osteoclast differentiation was evaluated using mouse bone marrow monocytes which were cultured with M-CSF and RANKL, then TRAP staining was performed to measure effect of hNTSCs on osteoclastogenesis. Microarray assays were performed to identify gene expression differences between hNTSCs with CIA mice therapeutic or not and were validated by RT-qPCR. hNTSCs differentiated well into osteoblasts and adipocytes and expressed high levels of CXCL1 and osteoprotegerin. Single-cell RNA sequencing showed that hNTSCs clustered into 11 cell types, and cell surface markers were compatible with mesenchymal stem cells. hNTSC-treated CIA mice showed reductions in arthritis severity scores and incidence of arthritis. In engraft measurements, hNTSCs survived for 8 to 12 weeks in mice paws. Chemokine receptors expression increased in hNTSCs by IL-1β or TNF-α stimulation. CD4+CD25- T cell proliferation was reduced by hNTSCs and reversed by adding 1-MT (indoleamine 2,3-dioxygenase inhibitor), indicating that indoleamine 2,3-dioxygenase mediated T cell suppression. Osteoclastogenesis was suppressed by hNTSCs, and this was attenuated by anti-OPG Ab. hNTSCs therapeutic in CIA mice showed specific gene signatures with up-regulated genes (KRTAP1-5, HAS2, and CXCL1) and down-regulated genes (GSTT2B and C4B) compared to hNTSCs without CIA therapeutic effects. hNTSCs exhibited therapeutic potential in RA. Therapeutic effects were mediated by effector helper T cell suppression and the inhibition of osteoclastogenesis. In addition, hNTSCs with greater therapeutic effects on RA showed significant differences in their gene signatures.
© 2025. The Author(s).
-
ICC-IF
-
FC/FACS
-
Homo sapiens (Human)
-
Stem Cells and Developmental Biology
Simple Isolation of Human Bone Marrow Adipose Tissue-Derived Mesenchymal Stem/Stromal Cells.
In Current Protocols on 1 January 2025 by Tonyalı, G., Kılıc, E., et al.
Bone marrow adipose tissue (BMAT) has garnered significant attention due to its critical roles in leukemia pathogenesis, cancer metastasis, and bone marrow failure. BMAT is a metabolically active, distinct tissue that differs from other fat depots. Marrow adipocytes, closely interacting with hematopoietic stem/progenitor cells and osteoblasts, play a pivotal role in regulating their functions. However, standardized methods for isolating and defining human BMAT (hBMAT) remain unclear. In animal models, BMAT is commonly isolated directly from the bone marrow through flushing, enzymatic digestion, or mechanical disruption. In humans, BMAT isolation often involves the adipogenic induction of bone marrow mesenchymal stem/stromal cells (BM-MSCs) derived from bone marrow aspirates. However, this approach reflects cellular responses to chemical stimuli and may not accurately represent in vivo differentiation potential. Similarly, BMAT obtained from hip or knee replacement surgeries might not reflect basal physiological conditions due to inflammatory influences. Here, we describe a direct method for culturing BMAT from the fatty layer of bone marrow aspirates obtained from healthy transplant donors. This protocol employs centrifugation and washing steps using basic laboratory equipment, offering simple and non-enzymatic approach. For validation, isolated cells are characterized according to the International Society for Cell & Gene Therapy (ISCT) criteria. © 2025 Wiley Periodicals LLC. Basic Protocol 1: Isolation of human BMAT-MSCs from the fatty layer of the bone marrow Basic Protocol 2: Culture expansion, trypsinization, and cryopreservation of BMAT-MSCs Support Protocol 1: Immunophenoypic characterization of human BMAT-MSCs by flow cytometry Support Protocol 2: In vitro characterization of multilineage differentiation potential of human BMAT-MSCs Support Protocol 3: Further characterization of gene expression in human BMAT-MSCs using qRT-PCR.
© 2025 Wiley Periodicals LLC.
In Biochemistry and Biophysics Reports on 1 December 2024 by Sun, B., Chen, H., et al.
PIK3CA-related overgrowth spectrum (PROS) encompasses several rare conditions that lead to overgrowth of various body parts resulting from activating variants in PIK3CA. The absence of ideal cell models significantly impedes progress in PROS research. In this study, we focused on facial infiltrating lipomatosis (FIL) (A disorder within PROS) and aimed to establish and characterize an immortalized PROS cell line. Primary adipose-derived stem cells of FIL were immortal-ized through the transfection of simian virus 40 large T antigen (SV40LT). No significant mor-phological differences were observed in immortalized FIL-ADSCs (Im FIL-ADSCs). Im FIL-ADSCs expressed original mesenchymal surface markers, confirmed by flow cytometry. It harbored PIK3CA mutation and an increased level of PI3K/AKT activation, revealed by sanger sequencing and Western blot respectively. Karyotype analysis revealed a stable chromosome in Im FIL-ADSCs. Higher adipogenic potential and lower osteogenic differentiation properties were de-tected in Im FIL-ADSCs. The proliferative potential of Im FIL-ADSCs increased, whereas malig-nant transformation was not observed in the tumorigenesis assay. Moreover, RNA sequencing further elucidated the role of the transcription factor E2F1 in Im FIL-ADSCs. Drug screening unveiled that STAT3, HSP, EGFR, and NF-kB might be potential therapeutic targets for FIL. This study provided a valuable cellular resource for exploring the underlying pathogenic mechanisms and developing new targeted therapeutic options for PROS.
© 2024 Published by Elsevier B.V.
-
FC/FACS
-
Stem Cells and Developmental Biology
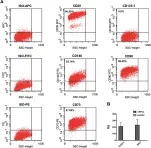
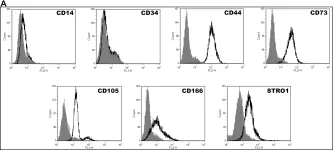
In J Transl Med on 14 August 2024 by Tanzi, A., Buono, L., et al.
Fig.3.A

-
FC/FACS
-
Homo sapiens (Human)
Collected and cropped from J Transl Med by CiteAb, provided under a CC-BY license
Image 1 of 9
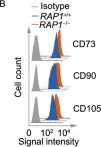
In Protein Cell on 1 September 2019 by Zhang, X., Liu, Z., et al.
Fig.2.B

-
FC/FACS
-
Homo sapiens (Human)
Collected and cropped from Protein Cell by CiteAb, provided under a CC-BY license
Image 1 of 9
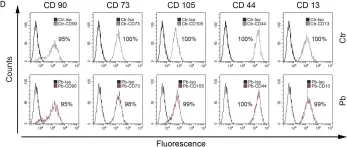
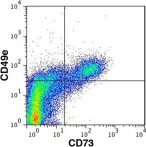
In PLoS One on 27 December 2018 by Griukova, A., Deryabin, P., et al.
Fig.1.D

-
FC/FACS
-
Homo sapiens (Human)
Collected and cropped from PLoS One by CiteAb, provided under a CC-BY license
Image 1 of 9
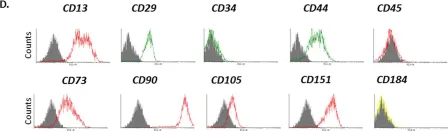
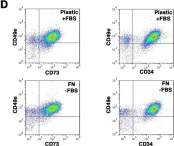
In Front Immunol on 17 May 2018 by Bastian, O. W., Croes, M., et al.
Fig.1.A

-
FC/FACS
-
Homo sapiens (Human)
Collected and cropped from Front Immunol by CiteAb, provided under a CC-BY license
Image 1 of 9
In NPJ Microgravity on 18 November 2017 by Weiss, W. M., Mulet-Sierra, A., et al.
Fig.1.D

-
FC/FACS
-
Homo sapiens (Human)
Collected and cropped from NPJ Microgravity by CiteAb, provided under a CC-BY license
Image 1 of 9
In Sci Rep on 14 March 2017 by Di Maggio, N., Martella, E., et al.
Fig.5.D
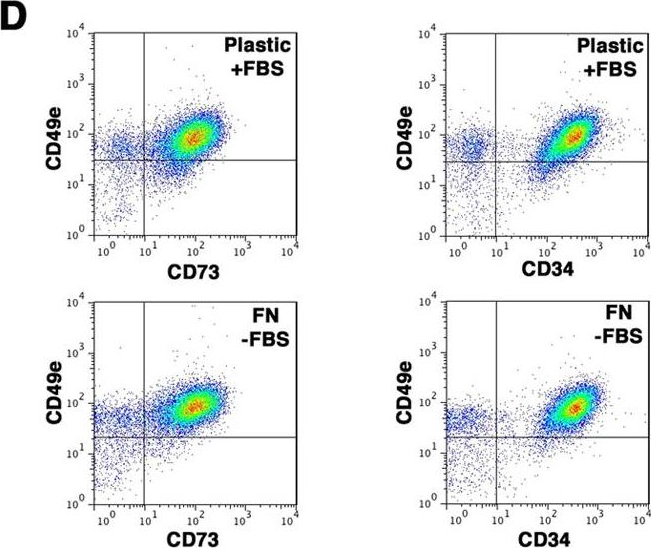
-
FC/FACS
-
Homo sapiens (Human)
Collected and cropped from Sci Rep by CiteAb, provided under a CC-BY license
Image 1 of 9
In Sci Rep on 14 March 2017 by Di Maggio, N., Martella, E., et al.
Fig.7.C

-
FC/FACS
-
Homo sapiens (Human)
Collected and cropped from Sci Rep by CiteAb, provided under a CC-BY license
Image 1 of 9
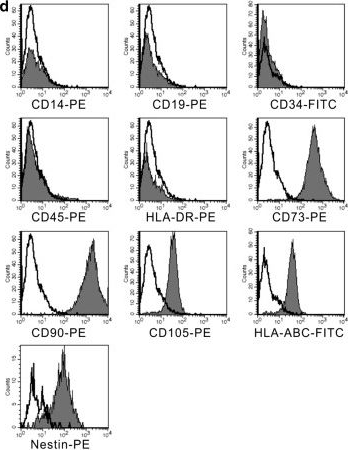
In Stem Cell Res Ther on 4 December 2014 by Wang, Y., Wu, H., et al.
Fig.2.D
-
FC/FACS
-
Homo sapiens (Human)
Collected and cropped from Stem Cell Res Ther by CiteAb, provided under a CC-BY license
Image 1 of 9
In PLoS One on 30 August 2014 by Tsai, H. L., Deng, W. P., et al.
Fig.1.A

-
FC/FACS
-
Homo sapiens (Human)
Collected and cropped from PLoS One by CiteAb, provided under a CC-BY license
Image 1 of 9