Bacterial multidrug resistance poses an urgent challenge for the treatment of critically ill patients developing ventilator-associated pneumonia (VAP). Phage therapy, a potential alternative when conventional antibiotics fail, has been unsuccessful in first clinical trials when used alone. Whether combining antibiotics with phages may enhance effectiveness remains to be tested in experimental models. Here, we use a murine model of Pseudomonas-induced VAP to compare the efficacy of adjunctive phage cocktail for antibiotic therapy to either meropenem or phages alone. Combined treatment in murine VAP results in faster clinical improvement and prevents lung epithelial cell damage. Using human primary epithelial cells to dissect these synergistic effects, we find that adjunctive phage therapy reduces the minimum effective concentration of meropenem and prevents resistance development against both treatments. These findings suggest adjunctive phage therapy represents a promising treatment for MDR-induced VAP, enhancing the effectiveness of both antibiotics and phages while reducing adverse effects.
© 2025. The Author(s).
Product Citations: 295
In Nature Communications on 15 May 2025 by Weissfuss, C., Li, J., et al.
-
Immunology and Microbiology
PD-1 blockade employed at the time CD8+ T cells are activated enhances their antitumor efficacy.
In Journal for Immunotherapy of Cancer on 7 May 2025 by Moseman, J. E., Rastogi, I., et al.
We have previously shown that immune checkpoint receptors, including PD-1, are upregulated on T cells at the time of their activation, and that blockade of these receptors can improve the efficacy of antitumor vaccines. In the present study, we sought to determine whether, and by what mechanisms, the timing of PD-1 blockade with respect to vaccination affects antitumor T cell function.
TRAMP-C1 or E.G7-OVA tumor-bearing mice received PD-1 blockade at different timing intervals with a tumor-associated antigen vaccine. Tumor growth, survival, and immune-infiltrating populations were assessed. In vitro models of T cell activation using OT-I T cells and PD-(L)1 axis disruption with a PD-1 blocking antibody or PD-L1KO dendritic cells were used.
Mice receiving PD-1 blockade at the time of T cell activation with vaccine had better antitumor outcomes in comparison to mice receiving PD-1 blockade before or after immunization. T cells activated in vitro in the presence of PD-(L)1 axis disruption had a more differentiated, functional phenotype with decreased CD28 and CCR7 expression and increased production of the Tc1 cytokines IL-2, TNFα, and IFNγ. Intriguingly, a small subset of undifferentiated cells (CD28+) was of a stem-like Tc17 phenotype (IL-17α+, TCF1+). Tumor-bearing mice receiving T cells activated in the presence of PD-(L)1-axis disruption had better antitumor outcomes and a greater number of complete responses.
These data indicate that PD-1 blockade, when used with antitumor vaccines, acts primarily at the time of T cell activation, not exclusively within the tumor microenvironment. Consequently, PD-1 blockade may be best used when delivered concurrently with T cell activating agents such as vaccines.
© Author(s) (or their employer(s)) 2025. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ Group.
-
Immunology and Microbiology
In Cellular and Molecular Life Sciences : CMLS on 30 March 2025 by Li, M., Wu, L., et al.
Macrophages play differential roles in the pathogenesis of atherosclerosis due to their different phenotypes. Although α-SMA+ macrophages have been found to present in bone marrow and atherosclerotic plaques, their role in atherosclerosis remains unclear. By performing partial carotid ligation (PCL) on monocyte/macrophage lineage-tracked mice, we observed bone marrow-derived α-SMA+ macrophages in the subendothelium and atherosclerotic plaques under disturbed flow conditions. The functional role of α-SMA+ macrophages in atherosclerotic plaque formation was examined using macrophage-specific Acta2 knockout (Acta2MKO) mice generated by crossing Acta2f/f transgenic mice with LysM-Cre mice. The size of the aortic plaques was 77.43% smaller in Acta2MKO mice than in Acta2f/f mice following adeno-associated virus-mutant PCSK9 injection and high-fat diet (HFD) feeding for 12 weeks. A significant reduction in lipid deposition, macrophage infiltration and the α-SMA+ area was observed in the aortic roots of Acta2MKO mice compared with Acta2f/f mice. Mechanistically, using Acta2-overexpressing Raw264.7 cells (Acta2hi cells) and bone marrow-derived macrophages (BMDMs) from Acta2MKO mice (Acta2MKO BMDMs), we showed that macrophage α-SMA increased the expression of the scavenger receptor SR-A, induced Ox-LDL binding and uptake, and reduced the level of the cholesterol transporter ABCA1, potentially via the AKT pathway. Together, our results indicate that bone marrow-derived α-SMA+ macrophages contribute to atherosclerotic plaque formation due to dysregulated cholesterol uptake and efflux, providing potential targets for the prevention and treatment of atherosclerosis.
© 2025. The Author(s).
-
Biochemistry and Molecular biology
IL-33-activated ILC2s induce tertiary lymphoid structures in pancreatic cancer.
In Nature on 1 February 2025 by Amisaki, M., Zebboudj, A., et al.
Tertiary lymphoid structures (TLSs) are de novo ectopic lymphoid aggregates that regulate immunity in chronically inflamed tissues, including tumours. Although TLSs form due to inflammation-triggered activation of the lymphotoxin (LT)-LTβ receptor (LTβR) pathway1, the inflammatory signals and cells that induce TLSs remain incompletely identified. Here we show that interleukin-33 (IL-33), the alarmin released by inflamed tissues2, induces TLSs. In mice, Il33 deficiency severely attenuates inflammation- and LTβR-activation-induced TLSs in models of colitis and pancreatic ductal adenocarcinoma (PDAC). In PDAC, the alarmin domain of IL-33 activates group 2 innate lymphoid cells (ILC2s) expressing LT that engage putative LTβR+ myeloid organizer cells to initiate tertiary lymphoneogenesis. Notably, lymphoneogenic ILC2s migrate to PDACs from the gut, can be mobilized to PDACs in different tissues and are modulated by gut microbiota. Furthermore, we detect putative lymphoneogenic ILC2s and IL-33-expressing cells within TLSs in human PDAC that correlate with improved prognosis. To harness this lymphoneogenic pathway for immunotherapy, we engineer a recombinant human IL-33 protein that expands intratumoural lymphoneogenic ILC2s and TLSs and demonstrates enhanced anti-tumour activity in PDAC mice. In summary, we identify the molecules and cells of a druggable pathway that induces inflammation-triggered TLSs. More broadly, we reveal a lymphoneogenic function for alarmins and ILC2s.
© 2025. The Author(s).
-
Cancer Research
In Cell Reports Medicine on 17 December 2024 by Shiga, Y., Rangel Olguin, A. G., et al.
Disruption of calcium (Ca2+) homeostasis in neurons is a hallmark of neurodegenerative diseases. Here, we investigate the mechanisms leading to Ca2+ dysregulation and ask whether altered Ca2+ dynamics impinge on neuronal stress and circuit dysfunction. Using two-photon microscopy, we show that ocular hypertension, a major risk factor in glaucoma, and optic nerve crush injury disrupt the capacity of retinal neurons to clear cytosolic Ca2+ leading to impaired light-evoked responses. Gene- and protein expression analysis reveal the loss of the sarco-endoplasmic reticulum (ER) Ca2+-ATPase2 pump (SERCA2/ATP2A2) in injured retinal neurons from mice and patients with primary open-angle glaucoma. Pharmacological activation or neuron-specific gene delivery of SERCA2 is sufficient to rescue single-cell Ca2+ dynamics and promote robust survival of damaged neurons. Furthermore, SERCA2 gene supplementation reduces ER stress, reestablishes circuit balance, and restores visual behaviors. Our findings reveal that enhancing the Ca2+ clearance capacity of vulnerable neurons alleviates organelle stress and promotes neurorecovery.
Copyright © 2024 The Authors. Published by Elsevier Inc. All rights reserved.
-
Cell Biology
In Nat Commun on 25 November 2022 by Yan, J., Zhang, Y., et al.
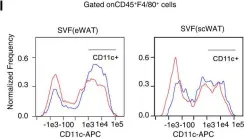
Fig.3.L

-
FC/FACS
-
Mus musculus (House mouse)
Collected and cropped from Nat Commun by CiteAb, provided under a CC-BY license
Image 1 of 8
In Nat Commun on 25 November 2022 by Yan, J., Zhang, Y., et al.
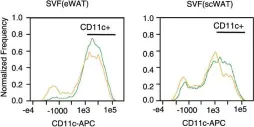
Fig.3.E

-
FC/FACS
-
Mus musculus (House mouse)
Collected and cropped from Nat Commun by CiteAb, provided under a CC-BY license
Image 1 of 8
In Nat Commun on 25 November 2022 by Yan, J., Zhang, Y., et al.
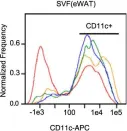
Fig.9.M

-
FC/FACS
-
Mus musculus (House mouse)
Collected and cropped from Nat Commun by CiteAb, provided under a CC-BY license
Image 1 of 8
In Nat Commun on 25 November 2022 by Yan, J., Zhang, Y., et al.
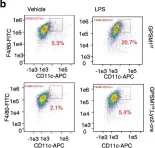
Fig.6.J

-
FC/FACS
-
Mus musculus (House mouse)
Collected and cropped from Nat Commun by CiteAb, provided under a CC-BY license
Image 1 of 8
In Nat Commun on 25 November 2022 by Yan, J., Zhang, Y., et al.
Fig.5.B

-
FC/FACS
-
Mus musculus (House mouse)
Collected and cropped from Nat Commun by CiteAb, provided under a CC-BY license
Image 1 of 8
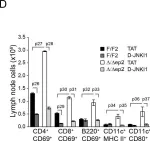
In Elife on 19 July 2016 by Raguz, J., Jerić, I., et al.
Fig.8.D

-
FC/FACS
-
Mus musculus (House mouse)
Collected and cropped from Elife by CiteAb, provided under a CC-BY license
Image 1 of 8
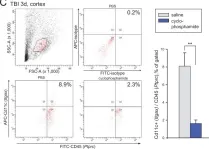
In PLoS One on 26 August 2014 by Israelsson, C., Kylberg, A., et al.
Fig.6.C

-
FC/FACS
-
Mus musculus (House mouse)
Collected and cropped from PLoS One by CiteAb, provided under a CC-BY license
Image 1 of 8
In PLoS One on 26 August 2014 by Israelsson, C., Kylberg, A., et al.
Fig.3.A

-
FC/FACS
-
Mus musculus (House mouse)
Collected and cropped from PLoS One by CiteAb, provided under a CC-BY license
Image 1 of 8