Impaired clearance of neurotoxic debris in the brain exacerbates neurologic disease and presents a promising therapeutic target. Pharmacologic therapies can enhance meningeal lymphatic clearance in preclinical models but may be limited by systemic toxicities or invasive administration. Here we report a low-intensity, focused ultrasound protocol that noninvasively clears pathogenic substances from the cerebrospinal fluid and brain interstitium in mice. Using two models of hemorrhagic stroke, we demonstrate that this protocol clears the cerebrospinal fluid and interstitium of blood cells, which accumulate in the deep cervical lymph nodes via meningeal lymphatics. The protocol directly modulates molecular processes, including mechanosensitive channels, to shift microglial phenotypes and astrocytic aquaporin localization to reduce neuroinflammation and neurocytotoxicity. In the intracerebral hemorrhage model, it improves behavioral outcomes and increases survival with greater efficacy than a pharmacologic benchmark. The protocol satisfies Food and Drug Administration safety guidelines, supporting clinical translatability. If demonstrated effective clinically, it may provide therapeutic benefit not only in hemorrhagic stroke but also in other neurologic disorders that involve impaired debris clearance.
© 2025. The Author(s).
Product Citations: 81
In Nature Biotechnology on 10 November 2025 by Azadian, M. M., Kiani Shabestari, S., et al.
-
IHC
-
Cardiovascular biology
-
Immunology and Microbiology
In Nature Communications on 27 March 2025 by Chen, H., Hu, K., et al.
Excessive cardiac fibrosis is a key cause of heart failure and adverse ventricular remodeling after myocardial infarction. The abnormally activated fibroblasts after scar maturation are the chief culprit. Single-cell RNA sequencing of mouse cardiac interstitial cells after myocardial infarction depicts a late-activated fibroblast subpopulation F-Act and initially identifies its characteristic antigen CD248, which is also verified in human hearts. On this basis, we develop a CD248-targeted biotin-binding immune receptor T cell therapy against F-Act to correct cardiac repair disorders. In our study, the precise removal of F-Act after the scar matured effectively inhibits fibrotic expansion in the peri-infarct zone and improves cardiac function. This therapy provides an idea for the treatment of cardiac fibrosis and also promotes the application of engineered T cells to non-tumor diseases.
© 2025. The Author(s).
-
Cardiovascular biology
-
Immunology and Microbiology
In Biomolecules on 23 October 2024 by Giacolone, J., Osofsky, R., et al.
Ischemic wounds are frequently encountered in clinical practice and may be related to ischemia secondary to diabetes, peripheral artery disease and other chronic conditions. Angiogenesis is critical to the resolution of ischemia. Hydrogen sulfide (H2S) is now recognized as an important factor in this process. H2S donors NaHS and GYY4137 were incorporated into the photosensitive polymer hydrogel gelatin methacrylate and evaluated. Human umbilical vein endothelial cell (HUVEC) culture was used to quantify toxicity and angiogenesis. Sprague Dawley rats were subjected to ischemic myocutaneous flap wound creation with and without application of H2S-eluting hydrogels. Tissue perfusion during wound healing was quantified using laser speckle contrast imaging, and gene and protein expression for VEGF were evaluated. Vascular density was assessed by CD31 immunohistochemistry. Successful incorporation of sulfide compounds was confirmed by scanning electron microscopy with energy-dispersive X-ray analysis, and under physiologic conditions, detectable H2S was present for up to 14 days by high-performance liquid chromatography. HUVECs exposed to hydrogels did not demonstrate excess cytotoxicity or apoptosis. A two-fold increase in angiogenic tube formation was observed in HUVECs exposed to H2S-eluting hydrogels. Rat ischemic flap wounds demonstrated greater perfusion at 14 days, and there was greater vascularity of healed wounds compared to untreated animals. A nearly two-fold increase in VEGF mRNA and a four-fold increase in VEGF protein expression were present in wounds from treated animals. Local-regional administration of H2S represents a novel potential therapeutic strategy to promote angiogenesis and improve wound healing after tissue injury or as a result of ischemic disease.
-
Biochemistry and Molecular biology
Indigo Leaves-Induced Pulmonary Arterial Remodeling without Right Ventricular Hypertrophy in Rats.
In Biological Pharmaceutical Bulletin on 1 August 2024 by Tsunematsu, H., Imanishi, M., et al.
Indigo naturalis (IN), derived from the leaves of the indigo plant, is a traditional Chinese medicine that has historically been used for its anti-inflammatory properties in the treatment of various diseases, including ulcerative colitis (UC). However, long-term use of IN in UC patients is incontrovertibly associated with the onset of pulmonary arterial hypertension (PAH). To investigate the mechanisms by which IN induces PAH, we focused on the raw material of IN, indigo leaves (IL). Only the condition of long-term chronic (6 months) and high-dose (containing 5% IL in the control diet) administration of IL induced medial thickening in the pulmonary arteries without right ventricular hypertrophy in our rat model. IL administration for a month did not induce pulmonary arterial remodeling but increased endothelin-1 (ET-1) expression levels within endothelial cell (EC) layers in the lungs. Gene Expression Omnibus analysis showed that ET-1 is a key regulator of PAH and that the IL component indican and its metabolite IS induced ET-1 mRNA expression via reactive oxygen species-dependent mechanism. We identified the roles of indican and IS in ET-1 expression in ECs, which were linked to pulmonary arterial remodeling in an animal model.
-
Cardiovascular biology
-
Plant Science
In International Journal of Molecular Sciences on 3 February 2024 by Vidal, L., Lopez-Garzon, M., et al.
Patellar tendinopathy is a common clinical problem, but its underlying pathophysiology remains poorly understood, primarily due to the absence of a representative experimental model. The most widely used method to generate such a model is collagenase injection, although this method possesses limitations. We developed an optimized rat model of patellar tendinopathy via the ultrasound-guided injection of collagenase mixed with a thermo-responsive Pluronic hydrogel into the patellar tendon of sixty male Wistar rats. All analyses were carried out at 3, 7, 14, 30, and 60 days post-injury. We confirmed that our rat model reproduced the pathophysiology observed in human patients through analyses of ultrasonography, histology, immunofluorescence, and biomechanical parameters. Tendons that were injured by the injection of the collagenase-Pluronic mixture exhibited a significant increase in the cross-sectional area (p < 0.01), a high degree of tissue disorganization and hypercellularity, significantly strong neovascularization (p < 0.01), important changes in the levels of types I and III collagen expression, and the organization and presence of intra-tendinous calcifications. Decreases in the maximum rupture force and stiffness were also observed. These results demonstrate that our model replicates the key features observed in human patellar tendinopathy. Collagenase is evenly distributed, as the Pluronic hydrogel prevents its leakage and thus, damage to surrounding tissues. Therefore, this model is valuable for testing new treatments for patellar tendinopathy.
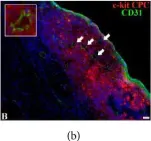
In Biomed Res Int on 27 July 2018 by Dergilev, K. V., Tsokolaeva, Z., et al.
Fig.5.B
-
IHC
-
Rattus norvegicus (Rat)
Collected and cropped from BioMed Research International by CiteAb, provided under a CC-BY license
Image 1 of 3
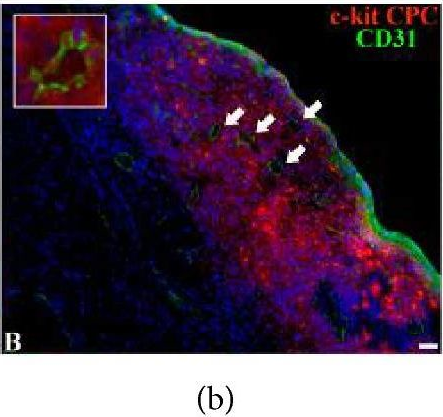
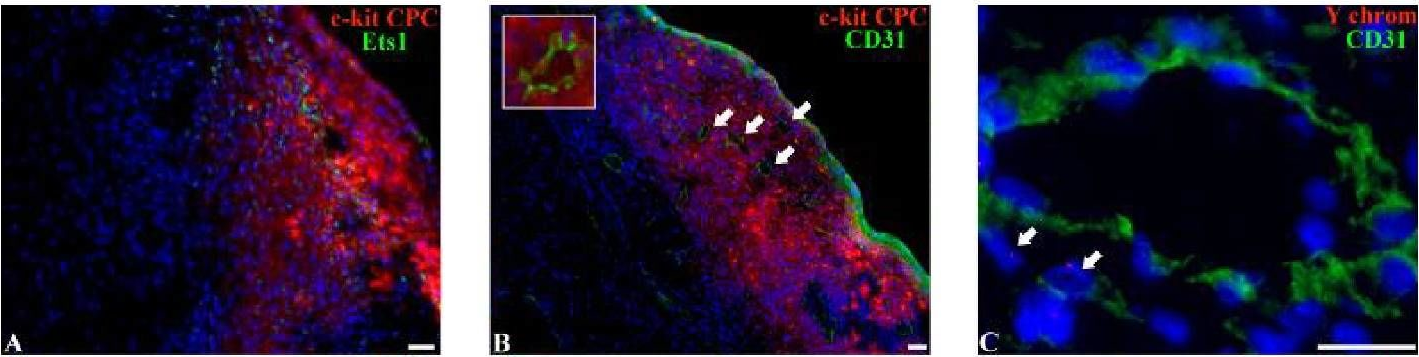
In Biomed Res Int on 27 July 2018 by Dergilev, K. V., Tsokolaeva, Z., et al.
Fig.5.C
-
IHC
-
Rattus norvegicus (Rat)
Collected and cropped from BioMed Research International by CiteAb, provided under a CC-BY license
Image 1 of 3
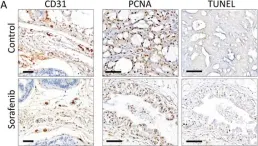
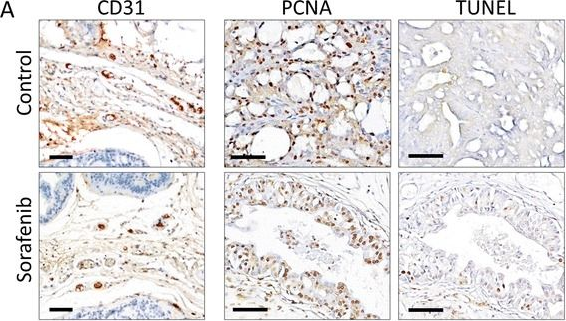
In J Transl Med on 8 May 2015 by Yamamoto, Y., De Velasco, M. A., et al.
Fig.3.A
-
IHC
-
Collected and cropped from Journal of Translational Medicine by CiteAb, provided under a CC-BY license
Image 1 of 3