Blood-brain barrier (BBB) dysfunction is a key feature of Alzheimer’s disease (AD), particularly in individuals carrying the APOE-ε4 allele. This dysfunction worsens neuroinflammation and hinders the removal of toxic proteins, such as amyloid-beta (Aβ42), from the brain. In post-mortem brain tissues and in animal models, we previously reported that fibronectin accumulates at the BBB predominantly in APOE-ε4 carriers. Furthermore, we found a loss-of-function variant in the fibronectin 1 ( FN1 ) gene significantly reduces aggregated fibronectin levels and decreases AD risk among APOE-ε4 carriers. Yet, the molecular mechanisms downstream of fibronectin at the BBB remain unclear. The extracellular matrix (ECM) plays a crucial role in maintaining BBB homeostasis and orchestrating the interactions between BBB cell types, including endothelia and astrocytes. Understanding the mechanisms affecting the ECM and BBB cell types will be critical for developing effective therapies against AD, especially among APOE-ε4 carriers. Here, we demonstrate that APOE-ε4 , Aβ42, and inflammation drive the induction of FN1 expression in several models including zebrafish, mice, iPSC-derived human 3D astrocyte and 3D cerebrovascular cell cultures, and in human brains. Fibronectin accumulation disrupts astroglial-endothelial interactions and the signalling cascade between vascular endothelial growth factor (VEGF), heparin-binding epidermal growth factor (HBEGF) and Insulin-like growth factor 1 (IGF1). This accumulation of fibronectin in APOE-ε4- associated AD potentiates BBB dysfunction, which strongly implicates reducing fibronectin deposition as a potential therapeutic target for AD. Graphical abstract Accessibility text This image illustrates the effects of different APOE isoforms (ApoE-ε3 and ApoE-ε4) on blood-brain barrier (BBB) integrity, focusing on the molecular interactions between astrocytes and endothelial cells. This figure emphasizes the detrimental effects of ApoE-ε4 on BBB integrity via fibronectin accumulation and altered signaling pathways. The top section provides a schematic overview of the blood-brain barrier, highlighting astrocytes, endothelial cells, and their interface. The left panel represents the ApoE-ε3 condition: Normal fibronectin (FN1) levels support healthy interactions between astrocytes and endothelial cells. Growth factors, including VEGFA, HBEGF, and IGF1, maintain BBB integrity through their respective receptors (VEGFR and EGFR). Green arrows indicate activation of these signaling pathways. The right panel depicts the ApoE-ε4 condition: Elevated fibronectin (FN1) disrupts astrocyte-endothelium interactions. FN1 binds integrins and activates focal adhesion kinase (FAK), inhibiting VEGFA, which is required for endothelial HBEGF that in turn activates IGF1 signaling. Red symbols indicate inhibition of HBEGF, VEGFA, and IGF1 pathways, leading to BBB dysfunction. Highlights APOE-ε4 drives fibronectin deposition in Alzheimer’s, disrupting astrocyte-endothelia interactions. APOE-ε4 and fibronectin co-localize, forming aggregates at blood-brain barrier (BBB). Fibronectin alters the signaling between VEGF, IGF1, and HBEGF impairing BBB function. Reducing fibronectin restores BBB integrity and offsets APOE-ε4 pathology.
Product Citations: 50
Preprint on BioRxiv : the Preprint Server for Biology on 27 January 2025 by Bhattarai, P., Yılmaz, E., et al.
-
Cardiovascular biology
-
Neuroscience
Molecules That Have Rarely Been Studied in Lymphatic Endothelial Cells.
In International Journal of Molecular Sciences on 14 November 2024 by Becker, J. & Wilting, J.
A number of standard molecules are used for the molecular and histological characterization of lymphatic endothelial cells (LECs), including lymphatic vessel endothelial hyaluronan receptor 1 (LYVE1), Podoplanin (D2-40), VEGFR3, Prospero homeobox protein 1 (PROX1), and CD31. The number of molecules whose mutations cause lymphatic malformations or primary congenital lymphedema is considerable, but the majority of these diseases have not yet been characterized at the molecular level. Therefore, there is still considerable scope for molecular and functional studies of the lymphatic vasculature. Using RNASeq, we have previously characterized lymphatic endothelial cells (LECs) under normoxic and hypoxic conditions. We used this information to compare it with immunohistochemical data. We carried out some of the immunohistology ourselves, and systematically studied the Human Protein Atlas, a cell and tissue database based in Sweden. Here we describe molecules that are expressed at RNA and protein levels in LECs, hoping to stimulate future functional studies of these molecules.
-
Homo sapiens (Human)
In Pain on 1 July 2024 by Mehling, K., Becker, J., et al.
Complex regional pain syndrome (CRPS) presents postinjury with disproportionate pain and neuropathic, autonomic, motor symptoms, and skin texture affection. However, the origin of these multiplex changes is unclear. Skin biopsies offer a window to analyze the somatosensory and vascular system as well as skin trophicity with their protecting barriers. In previous studies, barrier-protective exosomal microRNAs were altered in CRPS. We here postulated that tissue architecture and barrier proteins are already altered at the beginning of CRPS. We analyzed ipsilateral and contralateral skin biopsies of 20 fully phenotyped early CRPS patients compared with 20 age- and sex-matched healthy controls. We established several automated unbiased methods to comprehensively analyze microvessels and somatosensory receptors as well as barrier proteins, including claudin-1, claudin-5, and claudin-19. Meissner corpuscles in the skin were bilaterally reduced in acute CRPS patients with some of them lacking these completely. The number of Merkel cells and the intraepidermal nerve fiber density were not different between the groups. Dermal papillary microvessels were bilaterally less abundant in CRPS, especially in patients with allodynia. Barrier proteins in keratinocytes, perineurium of dermal nerves, Schwann cells, and papillary microvessels were not affected in early CRPS. Bilateral changes in the tissue architecture in early CRPS might indicate a predisposition for CRPS that manifests after injury. Further studies should evaluate whether these changes might be used to identify risk patients for CRPS after trauma and as biomarkers for outcome.
Copyright © 2024 The Author(s). Published by Wolters Kluwer Health, Inc. on behalf of the International Association for the Study of Pain.
-
Homo sapiens (Human)
Senescence of endplate osteoclasts induces sensory innervation and spinal pain.
In eLife on 19 June 2024 by Pan, D., Benkato, K. G., et al.
Spinal pain affects individuals of all ages and is the most common musculoskeletal problem globally. Its clinical management remains a challenge as the underlying mechanisms leading to it are still unclear. Here, we report that significantly increased numbers of senescent osteoclasts (SnOCs) are observed in mouse models of spinal hypersensitivity, like lumbar spine instability (LSI) or aging, compared to controls. The larger population of SnOCs is associated with induced sensory nerve innervation, as well as the growth of H-type vessels, in the porous endplate. We show that deletion of senescent cells by administration of the senolytic drug Navitoclax (ABT263) results in significantly less spinal hypersensitivity, spinal degeneration, porosity of the endplate, sensory nerve innervation, and H-type vessel growth in the endplate. We also show that there is significantly increased SnOC-mediated secretion of Netrin-1 and NGF, two well-established sensory nerve growth factors, compared to non-senescent OCs. These findings suggest that pharmacological elimination of SnOCs may be a potent therapy to treat spinal pain.
© 2023, Pan et al.
-
Neuroscience
In Molecular & Cellular Proteomics : MCP on 1 June 2024 by Enström, A., Carlsson, R., et al.
Cellular communication within the brain is imperative for maintaining homeostasis and mounting effective responses to pathological triggers like hypoxia. However, a comprehensive understanding of the precise composition and dynamic release of secreted molecules has remained elusive, confined primarily to investigations using isolated monocultures. To overcome these limitations, we utilized the potential of TurboID, a non-toxic biotin ligation enzyme, to capture and enrich secreted proteins specifically originating from human brain pericytes in spheroid cocultures with human endothelial cells and astrocytes. This approach allowed us to characterize the pericyte secretome within a more physiologically relevant multicellular setting encompassing the constituents of the blood-brain barrier. Through a combination of mass spectrometry and multiplex immunoassays, we identified a wide spectrum of different secreted proteins by pericytes. Our findings demonstrate that the pericytes secretome is profoundly shaped by their intercellular communication with other blood-brain barrier-residing cells. Moreover, we identified substantial differences in the secretory profiles between hypoxic and normoxic pericytes. Mass spectrometry analysis showed that hypoxic pericytes in coculture increase their release of signals related to protein secretion, mTOR signaling, and the complement system, while hypoxic pericytes in monocultures showed an upregulation in proliferative pathways including G2M checkpoints, E2F-, and Myc-targets. In addition, hypoxic pericytes show an upregulation of proangiogenic proteins such as VEGFA but display downregulation of canonical proinflammatory cytokines such as CXCL1, MCP-1, and CXCL6. Understanding the specific composition of secreted proteins in the multicellular brain microvasculature is crucial for advancing our knowledge of brain homeostasis and the mechanisms underlying pathology. This study has implications for the identification of targeted therapeutic strategies aimed at modulating microvascular signaling in brain pathologies associated with hypoxia.
Copyright © 2024 The Authors. Published by Elsevier Inc. All rights reserved.
-
Homo sapiens (Human)
-
Biochemistry and Molecular biology
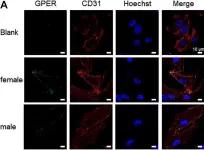
In Front Pharmacol on 5 January 2021 by Boscaro, C., Trenti, A., et al.
Fig.2.A

-
ICC-IF
-
Homo sapiens (Human)
Collected and cropped from Front Pharmacol by CiteAb, provided under a CC-BY license
Image 1 of 1