Pain after surgery causes significant suffering. Opioid analgesics cause severe side effects and accidental death. Therefore, there is an urgent need to develop non-opioid therapies for managing post-surgical pain. Local application of Clarix Flo (FLO), a human amniotic membrane (AM) product, attenuated established post-surgical pain hypersensitivity without exhibiting known side effects of opioid use in mice. This effect was achieved through direct inhibition of nociceptive dorsal root ganglion (DRG) neurons via CD44-dependent pathways. We further purified the major matrix component, the heavy chain-hyaluronic acid/pentraxin 3 (HC-HA/PTX3) from human AM that has greater purity and water solubility than FLO. HC-HA/PTX3 replicated FLO-induced neuronal and pain inhibition. Mechanistically, HC-HA/PTX3-induced cytoskeleton rearrangements to inhibit sodium current and high-voltage activated calcium current on nociceptive DRG neurons, suggesting it is a key bioactive component mediating pain relief. Collectively, our findings highlight the potential of naturally derived biologics from human birth tissues as an effective non-opioid treatment for post-surgical pain. Moreover, we unravel the underlying neuronal mechanisms of pain inhibition induced by FLO and HC-HA/PTX3.
© 2024, Zhang, Huang, Ford et al.
Product Citations: 309
Human birth tissue products as a non-opioid medicine to inhibit post-surgical pain.
In eLife on 13 December 2024 by Zhang, C., Huang, Q., et al.
Metabolic plasticity in a Pde6bSTOP/STOP retinitis pigmentosa mouse model following rescue.
In Molecular Metabolism on 1 October 2024 by Ayten, M., Díaz-Lezama, N., et al.
Retinitis pigmentosa (RP) is a hereditary retinal disease characterized by progressive photoreceptor degeneration, leading to vision loss. The best hope for a cure for RP lies in gene therapy. However, given that RP patients are most often diagnosed in the midst of ongoing photoreceptor degeneration, it is unknown how the retinal proteome changes as RP disease progresses, and which changes can be prevented, halted, or reversed by gene therapy.
Here, we used a Pde6b-deficient RP gene therapy mouse model and performed untargeted proteomic analysis to identify changes in protein expression during degeneration and after treatment.
We demonstrated that Pde6b gene restoration led to a novel form of homeostatic plasticity in rod phototransduction which functionally compensates for the decreased number of rods. By profiling protein levels of metabolic genes and measuring metabolites, we observed an upregulation of proteins associated with oxidative phosphorylation in mutant and treated photoreceptors.
In conclusion, the metabolic demands of the retina differ in our Pde6b-deficient RP mouse model and are not rescued by gene therapy treatment. These findings provide novel insights into features of both RP disease progression and long-term rescue with gene therapy.
Copyright © 2024 The Authors. Published by Elsevier GmbH.. All rights reserved.
-
Mus musculus (House mouse)
-
Biochemistry and Molecular biology
-
Cell Biology
CRACD loss induces neuroendocrine cell plasticity of lung adenocarcinoma.
In Cell Reports on 25 June 2024 by Kim, B., Zhang, S., et al.
Tumor cell plasticity contributes to intratumoral heterogeneity and therapy resistance. Through cell plasticity, some lung adenocarcinoma (LUAD) cells transform into neuroendocrine (NE) tumor cells. However, the mechanisms of NE cell plasticity remain unclear. CRACD (capping protein inhibiting regulator of actin dynamics), a capping protein inhibitor, is frequently inactivated in cancers. CRACD knockout (KO) is sufficient to de-repress NE-related gene expression in the pulmonary epithelium and LUAD cells. In LUAD mouse models, Cracd KO increases intratumoral heterogeneity with NE gene expression. Single-cell transcriptomic analysis showed that Cracd KO-induced NE cell plasticity is associated with cell de-differentiation and stemness-related pathway activation. The single-cell transcriptomic analysis of LUAD patient tumors recapitulates that the distinct LUAD NE cell cluster expressing NE genes is co-enriched with impaired actin remodeling. This study reveals the crucial role of CRACD in restricting NE cell plasticity that induces cell de-differentiation of LUAD.
Copyright © 2024 The Authors. Published by Elsevier Inc. All rights reserved.
-
Cancer Research
-
Endocrinology and Physiology
Preprint on BioRxiv : the Preprint Server for Biology on 20 May 2024 by Zhang, C., Huang, Q., et al.
Pain after surgery causes significant suffering. Opioid analgesics cause severe side effects and accidental death. Therefore, there is an urgent need to develop non-opioid therapies for managing post-surgical pain and, more importantly, preventing its transition to a chronic state. In a mouse model of post-surgical pain, local application of Clarix Flo (FLO), a human amniotic membrane (AM) product, attenuated established post-surgical pain hypersensitivity without exhibiting known side effects of opioid use in mice. Importantly, preemptive drug treatment also inhibited the transition of post-surgical pain to a prolonged state. This effect was achieved through direct inhibition of nociceptive dorsal root ganglion (DRG) neurons via CD44-dependent pathways, and indirect pain relief by attenuating immune cell recruitment. We further purified the major matrix component, the heavy chain-hyaluronic acid/pentraxin 3 (HC-HA/PTX3) from human AM that has greater purity and water solubility than FLO. HC-HA/PTX3 replicated FLO- induced neuronal and pain inhibition. Mechanistically, HC-HA/PTX3 induced cytoskeleton rearrangements to inhibit sodium current and high-voltage activated calcium current on nociceptive neurons, suggesting it is a key bioactive component mediating pain relief. Collectively, our findings highlight the potential of naturally derived biologics from human birth tissues as an effective non-opioid treatment for post-surgical pain and unravel the underlying mechanisms.
-
ICC-IF
-
Mus musculus (House mouse)
In Biomedicines on 30 April 2024 by Fabritz, L., Fortmueller, L., et al.
Desmoglein-2 mutations are detected in 5-10% of patients with arrhythmogenic right ventricular cardiomyopathy (ARVC). Endurance training accelerates the development of the ARVC phenotype, leading to earlier arrhythmic events. Homozygous Dsg2 mutant mice develop a severe ARVC-like phenotype. The phenotype of heterozygous mutant (Dsg2mt/wt) or haploinsufficient (Dsg20/wt) mice is still not well understood. To assess the effects of age and endurance swim training, we studied cardiac morphology and function in sedentary one-year-old Dsg2mt/wt and Dsg20/wt mice and in young Dsg2mt/wt mice exposed to endurance swim training. Cardiac structure was only occasionally affected in aged Dsg20/wt and Dsg2mt/wt mice manifesting as small fibrotic foci and displacement of Connexin 43. Endurance swim training increased the right ventricular (RV) diameter and decreased RV function in Dsg2mt/wt mice but not in wild types. Dsg2mt/wt hearts showed increased ventricular activation times and pacing-induced ventricular arrhythmia without obvious fibrosis or inflammation. Preload-reducing therapy during training prevented RV enlargement and alleviated the electrophysiological phenotype. Taken together, endurance swim training induced features of ARVC in young adult Dsg2mt/wt mice. Prolonged ventricular activation times in the hearts of trained Dsg2mt/wt mice are therefore a potential mechanism for increased arrhythmia risk. Preload-reducing therapy prevented training-induced ARVC phenotype pointing to beneficial treatment options in human patients.
-
IHC
-
Mus musculus (House mouse)
-
Cardiovascular biology
In J Clin Invest on 16 August 2021 by Veatch, J. R., Singhi, N., et al.
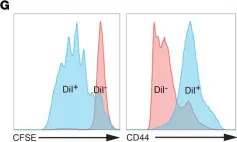
Fig.2.G

-
FC/FACS
-
Homo sapiens (Human)
Collected and cropped from J Clin Invest by CiteAb, provided under a CC-BY license
Image 1 of 4
In J Clin Invest on 16 August 2021 by Veatch, J. R., Singhi, N., et al.
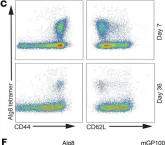
Fig.4.A

-
FC/FACS
-
Homo sapiens (Human)
Collected and cropped from J Clin Invest by CiteAb, provided under a CC-BY license
Image 1 of 4
In Elife on 18 June 2018 by Bettonville, M., d'Aria, S., et al.
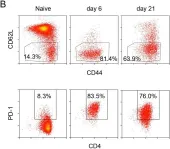
Fig.1.B

-
FC/FACS
-
Collected and cropped from Elife by CiteAb, provided under a CC-BY license
Image 1 of 4
In Elife on 18 June 2018 by Bettonville, M., d'Aria, S., et al.
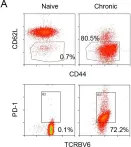
Fig.2.A

-
FC/FACS
-
Collected and cropped from Elife by CiteAb, provided under a CC-BY license
Image 1 of 4