Metastasis is the primary cause of cancer mortality, and cancer frequently metastasizes to the liver. It is not clear whether liver immune tolerance mechanisms contribute to cancer outcomes. We report that liver metastases diminish immunotherapy efficacy systemically in patients and preclinical models. Patients with liver metastases derive limited benefit from immunotherapy independent of other established biomarkers of response. In multiple mouse models, we show that liver metastases siphon activated CD8+ T cells from systemic circulation. Within the liver, activated antigen-specific Fas+CD8+ T cells undergo apoptosis following their interaction with FasL+CD11b+F4/80+ monocyte-derived macrophages. Consequently, liver metastases create a systemic immune desert in preclinical models. Similarly, patients with liver metastases have reduced peripheral T cell numbers and diminished tumoral T cell diversity and function. In preclinical models, liver-directed radiotherapy eliminates immunosuppressive hepatic macrophages, increases hepatic T cell survival and reduces hepatic siphoning of T cells. Thus, liver metastases co-opt host peripheral tolerance mechanisms to cause acquired immunotherapy resistance through CD8+ T cell deletion, and the combination of liver-directed radiotherapy and immunotherapy could promote systemic antitumor immunity.
Product Citations: 68
Liver metastasis restrains immunotherapy efficacy via macrophage-mediated T cell elimination.
In Nature Medicine on 1 January 2021 by Yu, J., Green, M. D., et al.
-
Cancer Research
-
Immunology and Microbiology
Enhancing mucosal immunity by transient microbiota depletion.
In Nature Communications on 8 September 2020 by Becattini, S., Littmann, E. R., et al.
Tissue resident memory CD8+ T cells (Trm) are poised for immediate reactivation at sites of pathogen entry and provide optimal protection of mucosal surfaces. The intestinal tract represents a portal of entry for many infectious agents; however, to date specific strategies to enhance Trm responses at this site are lacking. Here, we present TMDI (Transient Microbiota Depletion-boosted Immunization), an approach that leverages antibiotic treatment to temporarily restrain microbiota-mediated colonization resistance, and favor intestinal expansion to high densities of an orally-delivered Listeria monocytogenes strain carrying an antigen of choice. By augmenting the local chemotactic gradient as well as the antigenic load, this procedure generates a highly expanded pool of functional, antigen-specific intestinal Trm, ultimately enhancing protection against infectious re-challenge in mice. We propose that TMDI is a useful model to dissect the requirements for optimal Trm responses in the intestine, and also a potential platform to devise novel mucosal vaccination approaches.
-
Immunology and Microbiology
Generation of self-organized sensory ganglion organoids and retinal ganglion cells from fibroblasts.
In Science Advances on 1 May 2020 by Xiao, D., Deng, Q., et al.
Neural organoids provide a powerful tool for investigating neural development, modeling neural diseases, screening drugs, and developing cell-based therapies. Somatic cells have previously been reprogrammed by transcription factors (TFs) into sensory ganglion (SG) neurons but not SG organoids. We identify a combination of triple TFs Ascl1, Brn3b/3a, and Isl1 (ABI) as an efficient means to reprogram mouse and human fibroblasts into self-organized and networked induced SG (iSG) organoids. The iSG neurons exhibit molecular features, subtype diversity, electrophysiological and calcium response properties, and innervation patterns characteristic of peripheral sensory neurons. Moreover, we have defined retinal ganglion cell (RGC)-specific identifiers to demonstrate the ability for ABI to reprogram induced RGCs (iRGCs) from fibroblasts. Unlike iSG neurons, iRGCs maintain a scattering distribution pattern characteristic of endogenous RGCs. iSG organoids may serve as a model to decipher the pathogenesis of sensorineural diseases and screen effective drugs and a source for cell replacement therapy.
Copyright © 2020 The Authors, some rights reserved; exclusive licensee American Association for the Advancement of Science. No claim to original U.S. Government Works. Distributed under a Creative Commons Attribution NonCommercial License 4.0 (CC BY-NC).
-
Mus musculus (House mouse)
-
Neuroscience
In Immune Network on 1 August 2019 by Son, J. & Ha, S. J.
CD80 is mainly expressed on Ag-presenting cells (APCs) as a costimulatory molecule but is also detected on T cells. However, the origin and physiological role of CD80 on CD8+ T cells remain unclear. In the present study, we demonstrated that effector and memory CD8+ T cells, but not naïve CD8+ T cells, displayed CD80 molecules on their surfaces after acute lymphocytic choriomeningitis virus infection. Using adoptive transfer of CD80-knockout (KO) CD8+ T cells into a wild type or CD80-KO recipient, we demonstrated that the effector CD8+ T cells displayed CD80 by both intrinsic expression and extrinsic acquisition, while memory CD8+ T cells displayed CD80 only by extrinsic acquisition. Interestingly, the extrinsic acquisition of CD80 by CD8+ T cells was observed only in the lymphoid organs but not in the periphery, indicating the trogocytosis of CD80 molecules via interaction between CD8+ T cells and APCs. We compared the recall immune responses by memory CD8+ T cells that either extrinsically acquired CD80 or were deficient in CD80, and found that CD80, presented by memory CD8+ T cells, played a role in limiting their expansion and IL-2 production upon exposure to secondary challenge. Our study presents the in vivo dynamics of the extrinsic acquisition of CD80 by Ag-specific CD8+ T cells and its role in the regulation of recall immune responses in memory CD8+ T cells.
-
Immunology and Microbiology
Nrf2 controls iron homeostasis in haemochromatosis and thalassaemia via Bmp6 and hepcidin.
In Nature Metabolism on 1 May 2019 by Lim, P. J., Duarte, T. L., et al.
Iron is critical for life but toxic in excess because of iron-catalysed formation of pro-oxidants that cause tissue damage in a range of disorders. The Nrf2 transcription factor orchestrates cell-intrinsic protective antioxidant responses, and the peptide hormone hepcidin maintains systemic iron homeostasis, but is pathophysiologically decreased in haemochromatosis and beta-thalassaemia. Here, we show that Nrf2 is activated by iron-induced, mitochondria-derived pro-oxidants and drives Bmp6 expression in liver sinusoid endothelial cells, which in turn increases hepcidin synthesis by neighbouring hepatocytes. In Nrf2 knockout mice, the Bmp6-hepcidin response to oral and parenteral iron is impaired and iron accumulation and hepatic damage are increased. Pharmacological activation of Nrf2 stimulates the Bmp6-hepcidin axis, improving iron homeostasis in haemochromatosis and counteracting the inhibition of Bmp6 by erythroferrone in beta-thalassaemia. We propose that Nrf2 links cellular sensing of excess toxic iron to control of systemic iron homeostasis and antioxidant responses, and may be a therapeutic target for iron-associated disorders.
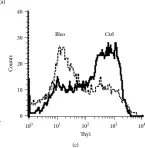
In Mediators Inflamm on 25 August 2015 by Cohen, P. Y., Breuer, R., et al.
Fig.1.A

-
FC/FACS
-
Mus musculus (House mouse)
Collected and cropped from Mediators Inflamm by CiteAb, provided under a CC-BY license
Image 1 of 1