Peripheral nerve injuries can lead to lasting functional impairments, impacting movement and quality of life. FK-506, a widely used immunosuppressant, has demonstrated potential in promoting nerve regeneration in addition to its immunosuppressive effects. This study investigates the use of a local reservoir flap to deliver FK-506 directly to the nerve injury site, aiming to enhance nerve regeneration while minimizing systemic immunosuppression.
Sciatic nerve injuries were surgically induced in 24 rats, which were divided into control, 0.5 mg/kg FK-506 (Exp 1), and 2.0 mg/kg FK-506 (Exp 2) groups. A superficial inferior epigastric artery flap served as a reservoir for FK-506, allowing direct delivery to the injury site. FK-506 was administered intermittently over a 4-week period. Outcomes included the Sciatic Functional Index (SFI), muscle recovery (width and weight), nerve morphology, expression of neurogenic markers such as GDNF, immune cell counts, and body weight.
Exp 1 (0.5 mg/kg) demonstrated significant improvements in SFI, GDNF expression, and muscle width compared to the control and high-dose groups. These findings suggest that FK-506 administration via a reservoir flap, particularly at a lower dose, supports effective nerve regeneration. Additionally, FK-506 treatment did not result in significant changes in immune cell profiles or body weight, indicating minimal systemic effects.
Localized FK-506 administration via a reservoir flap effectively enhances peripheral nerve regeneration and minimizes systemic immunosuppression, making it a promising approach for clinical application in treating peripheral nerve injuries.
© Copyright: Yonsei University College of Medicine 2024.
Product Citations: 136
In Yonsei Medical Journal on 1 December 2024 by Hong, J. W., Lim, J. H., et al.
-
Neuroscience
In Nature Communications on 18 September 2024 by Pala, Z. R., Alves e Silva, T. L., et al.
The evolution of hematophagy involves a series of adaptations that allow blood-feeding insects to access and consume blood efficiently while managing and circumventing the host's hemostatic and immune responses. Mosquito, and other insects, utilize salivary proteins to regulate these responses at the bite site during and after blood feeding. We investigated the function of Anopheles gambiae salivary apyrase (AgApyrase) in regulating hemostasis in the mosquito blood meal and in Plasmodium transmission. Our results demonstrate that salivary apyrase, a known inhibitor of platelet aggregation, interacts with and activates tissue plasminogen activator, facilitating the conversion of plasminogen to plasmin, a human protease that degrades fibrin and facilitates Plasmodium transmission. We show that mosquitoes ingest a substantial amount of apyrase during blood feeding, which reduces coagulation in the blood meal by enhancing fibrin degradation and inhibiting platelet aggregation. AgApyrase significantly enhanced Plasmodium infection in the mosquito midgut, whereas AgApyrase immunization inhibited Plasmodium mosquito infection and sporozoite transmission. This study highlights a pivotal role for mosquito salivary apyrase for regulation of hemostasis in the mosquito blood meal and for Plasmodium transmission to mosquitoes and to the mammalian host, underscoring the potential for strategies to prevent malaria transmission.
© 2024. This is a U.S. Government work and not under copyright protection in the US; foreign copyright protection may apply.
-
Mus musculus (House mouse)
-
Cardiovascular biology
Jak2V617F Reversible Activation Shows Its Essential Requirement in Myeloproliferative Neoplasms.
In Cancer Discovery on 1 May 2024 by Dunbar, A., Bowman, R. L., et al.
Gain-of-function mutations activating JAK/STAT signaling are seen in the majority of patients with myeloproliferative neoplasms (MPN), most commonly JAK2V617F. Although clinically approved JAK inhibitors improve symptoms and outcomes in MPNs, remissions are rare, and mutant allele burden does not substantively change with chronic therapy. We hypothesized this is due to limitations of current JAK inhibitors to potently and specifically abrogate mutant JAK2 signaling. We therefore developed a conditionally inducible mouse model allowing for sequential activation, and then inactivation, of Jak2V617F from its endogenous locus using a combined Dre-rox/Cre-lox dual-recombinase system. Jak2V617F deletion abrogates MPN features, induces depletion of mutant-specific hematopoietic stem/progenitor cells, and extends overall survival to an extent not observed with pharmacologic JAK inhibition, including when cooccurring with somatic Tet2 loss. Our data suggest JAK2V617F represents the best therapeutic target in MPNs and demonstrate the therapeutic relevance of a dual-recombinase system to assess mutant-specific oncogenic dependencies in vivo.
Current JAK inhibitors to treat myeloproliferative neoplasms are ineffective at eradicating mutant cells. We developed an endogenously expressed Jak2V617F dual-recombinase knock-in/knock-out model to investigate Jak2V617F oncogenic reversion in vivo. Jak2V617F deletion abrogates MPN features and depletes disease-sustaining MPN stem cells, suggesting improved Jak2V617F targeting offers the potential for greater therapeutic efficacy. See related commentary by Celik and Challen, p. 701. This article is featured in Selected Articles from This Issue, p. 695.
©2024 The Authors; Published by the American Association for Cancer Research.
-
IHC
-
Mus musculus (House mouse)
-
Cancer Research
Effect of erythrophagocytosis-induced ferroptosis during angiogenesis in atherosclerotic plaques.
In Angiogenesis on 1 November 2023 by Puylaert, P., Roth, L., et al.
Intraplaque (IP) angiogenesis is a key feature of advanced atherosclerotic plaques. Because IP vessels are fragile and leaky, erythrocytes are released and phagocytosed by macrophages (erythrophagocytosis), which leads to high intracellular iron content, lipid peroxidation and cell death. In vitro experiments showed that erythrophagocytosis by macrophages induced non-canonical ferroptosis, an emerging type of regulated necrosis that may contribute to plaque destabilization. Erythrophagocytosis-induced ferroptosis was accompanied by increased expression of heme-oxygenase 1 and ferritin, and could be blocked by co-treatment with third generation ferroptosis inhibitor UAMC-3203. Both heme-oxygenase 1 and ferritin were also expressed in erythrocyte-rich regions of carotid plaques from ApoE-/- Fbn1C1039G+/- mice, a model of advanced atherosclerosis with IP angiogenesis. The effect of UAMC-3203 (12.35 mg/kg/day) on atherosclerosis was evaluated in ApoE-/- Fbn1C1039G+/- mice fed a western-type diet (WD) for 12 weeks (n = 13 mice/group) or 20 weeks (n = 16-21 mice/group) to distinguish between plaques without and with established IP angiogenesis, respectively. A significant decrease in carotid plaque thickness was observed after 20 weeks WD (87 ± 19 μm vs. 166 ± 20 μm, p = 0.006), particularly in plaques with confirmed IP angiogenesis or hemorrhage (108 ± 35 μm vs. 322 ± 40 μm, p = 0.004). This effect was accompanied by decreased IP heme-oxygenase 1 and ferritin expression. UAMC-3203 did not affect carotid plaques after 12 weeks WD or plaques in the aorta, which typically do not develop IP angiogenesis. Altogether, erythrophagocytosis-induced ferroptosis during IP angiogenesis leads to larger atherosclerotic plaques, an effect that can be prevented by ferroptosis inhibitor UAMC-3203.
© 2023. The Author(s).
-
IHC
-
Mus musculus (House mouse)
-
Cardiovascular biology
BCRP drives intrinsic chemoresistance in chemotherapy-naïve breast cancer brain metastasis.
In Science Advances on 20 October 2023 by Uceda-Castro, R., Margarido, A. S., et al.
Although initially successful, treatments with chemotherapy often fail because of the recurrence of chemoresistant metastases. Since these tumors develop after treatment, resistance is generally thought to occur in response to chemotherapy. However, alternative mechanisms of intrinsic chemoresistance in the chemotherapy-naïve setting may exist but remain poorly understood. Here, we study drug-naïve murine breast cancer brain metastases (BCBMs) to identify how cancer cells growing in a secondary site can acquire intrinsic chemoresistance without cytotoxic agent exposure. We demonstrate that drug-naïve murine breast cancer cells that form cancer lesions in the brain undergo vascular mimicry and concomitantly express the adenosine 5'-triphosphate-binding cassette transporter breast cancer resistance protein (BCRP), a common marker of brain endothelial cells. We reveal that expression of BCRP by the BCBM tumor cells protects them against doxorubicin and topotecan. We conclude that BCRP overexpression can cause intrinsic chemoresistance in cancer cells growing in metastatic sites without prior chemotherapy exposure.
-
Mus musculus (House mouse)
-
Cancer Research
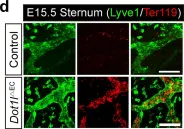
In Cell Death Dis on 6 January 2020 by Yoo, H., Lee, Y. J., et al.
Fig.1.D

-
IHC
-
Mus musculus (House mouse)
Collected and cropped from Cell Death Dis by CiteAb, provided under a CC-BY license
Image 1 of 1