Acute myeloid leukemia (AML) is the most prevalent form of leukemia in adults. The cornerstone of first‑line chemotherapy for AML has poor survival rates, underscoring the urgent need for development of novel therapeutic agents. Differentiation therapy targets the blockade of differentiation in myeloid progenitor cells. The present study screened 100 plant extracts native to South Korea to search for those with differentiation‑inducing activity in AML. Differentiation‑inducing activity was assessed by measuring CD11b expression using fluorescence activated cell sorting. Of these, Corydalis incisa (Thunb.) Pers. (CIP) exhibited the highest efficacy. CIP induced myeloid differentiation, decreased viability and increased cell apoptosis and cell cycle arrest in HL‑60, U937 and THP‑1 cells. Furthermore, ultra‑performance liquid chromatography‑quadrupole time‑of‑flight mass spectrometry identified norchelerythrine as the primary anti‑leukemic compound in CIP. Norchelerythrine induced differentiation and promoted cell cycle arrest and apoptosis, mirroring the tumor‑suppressive effects of CIP, and notably decreased cell viability in patients with various genetic abnormalities. The present mechanistic study showed that norchelerythrine stimulated reactive oxygen species generation, leading to activation of DNA damage signaling and upregulation of p21cip1, a cyclin‑dependent kinase inhibitor. Overall, norchelerythrine isolated from CIP may be a novel therapeutic option in AML.
Product Citations: 41
In International Journal of Oncology on 1 March 2025 by Lee, J. E., Jeon, B. E., et al.
-
Cancer Research
-
Genetics
In International Journal of Molecular Sciences on 5 February 2025 by Kwon, C. S., Jeon, B. E., et al.
Acute myeloid leukemia (AML) is characterized by the accumulation of immature myeloid cells and a differentiation block, highlighting the urgent need for novel differentiation-inducing therapies. This study evaluated Adina rubella Hance (ARH) stem as a potent differentiation inducer by systematically screening 200 plant extracts. ARH stem promoted phenotypic differentiation in AML cells. In addition to its differentiation-inducing effects, ARH stem exhibited strong antileukemic activities, such as inhibiting cell proliferation, inducing cell death, and enhancing mitochondrial reactive oxygen species (mtROS) levels, the latter of which is critical for its differentiation-promoting activity. Comparative analysis with the extracts from other parts of the plant confirmed the superior efficacy of the stem extract because of its unique chemical composition. Ultra-high-performance liquid chromatography combined with quadrupole time-of-flight mass spectrometry analysis identified Picroside III as a major active compound within the stem extract, capable of recapitulating ARH stem-induced differentiation and demonstrating significant antileukemic properties. These findings underscore the therapeutic potential of ARH stem and its active component, Picroside III, as promising agents for differentiation-based treatment strategies in AML.
-
Cell Biology
In Physiological Reports on 1 November 2024 by Imtiyaz, Z., O'Neill, O. J., et al.
Information is scarce on human responses to high pressure exposures out of water, such as related to tunnel construction workers. We hypothesized that differences in the longer durations of exposures for tunnel workers versus underwater divers results in greater inflammatory responses linked to the pathophysiology of decompression sickness (DCS). Blood was analyzed from 15 tunnel workers (36.1 ± 10.5 (SD) years old, 6 women) exposed to 142-156 kPa pressure for 4.1-4.9 h compared to 8 SCUBA divers (39.3 ± 13.3 (SD) years old, 6 women) exposed to 149 kPa for 0.61 hours. Despite differences in pressure duration between groups, elevations were the same for blood microparticles (MPs) (128 ± 28% MPs/μl) and intra-MPs interleukin (IL-1β) (376 ± 212% pg/million MPs), and for decreases of plasma gelsolin (pGSN, 31 ± 27% μg/mL). The number of circulating CD66b + neutrophils and evidence of cell activation, insignificant for divers, increased in tunnel workers. Across 3 exposures, the mean neutrophil count increased 150 ± 11%. Neutrophil activation increased by 1 to 2% of cells expressing cell surface CD18, myeloperoxidase, platelet-specific CD41, and decrease of cell bound pGSN. We conclude that MPs elevations occur rapidly in humans and reach steady state in minutes with pressure exposures and neutrophil activation requires significantly longer exposure times.
© 2024 The Author(s). Physiological Reports published by Wiley Periodicals LLC on behalf of The Physiological Society and the American Physiological Society.
-
Immunology and Microbiology
In Frontiers in Immunology on 30 October 2023 by Bazargan, S., Bunch, B., et al.
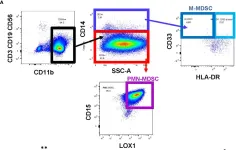
New therapeutics in development for bladder cancer need to address the recalcitrant nature of the disease. Intravesical adoptive cell therapy (ACT) with tumor infiltrating lymphocytes (TIL) can potentially induce durable responses in bladder cancer while maximizing T cells at the tumor site. T cells infused into the bladder directly encounter immunosuppressive populations, such as myeloid derived suppressor cells (MDSCs), that can attenuate T cell responses. Intravesical instillation of gemcitabine can be used as a lymphodepleting agent to precondition the bladder microenvironment for infused T cell products.
Urine samples from bladder cancer patients and healthy donors were analyzed by flow cytometry and cytometric bead array for immune profiling and cytokine quantification. MDSCs were isolated from the urine and cocultured with stimulated T cells to assess effects on proliferation. An orthotopic murine model of bladder cancer was established using the MB49-OVA cell line and immune profiling was performed. MDSCs from tumor-bearing mice were cocultured with OT-I splenocytes to assess T cell proliferation. Mice received intravesical instillation of gemcitabine and depletion of immune cells was measured via flow cytometry. Bladder tumor growth of mice treated with intravesical gemcitabine, OT-I transgenic T cells, or combination was monitored via ultrasound measurement.
In comparison to healthy donors, urine specimen from bladder cancer patients show high levels of MDSCs and cytokines associated with myeloid chemotaxis, T cell chemotaxis, and inflammation. T cells isolated from healthy donors were less proliferative when cocultured with MDSCs from the urine. Orthotopic murine bladder tumors also presented with high levels of MDSCs along with enrichment of cytokines found in the patient urine samples. MDSCs isolated from spleens of tumor-bearing mice exerted suppressive effects on the proliferation of OT-I T cells. Intravesical instillation of gemcitabine reduced overall immune cells, MDSCs, and T cells in orthotopic bladder tumors. Combination treatment with gemcitabine and OT-I T cells resulted in sustained anti-tumor responses in comparison to monotherapy treatments.
MDSCs are enriched within the microenvironment of bladder tumors and are suppressive to T cells. Gemcitabine can be used to lymphodeplete bladder tumors and precondition the microenvironment for intravesical ACT.
Copyright © 2023 Bazargan, Bunch, Ojwang‘, Blauvelt, Landin, Ali, Abrahams, Cox, Hall, Beatty, Poch, Rejniak and Pilon-Thomas.
-
FC/FACS
-
Homo sapiens (Human)
-
Cancer Research
-
Immunology and Microbiology
In Frontiers in Cellular and Infection Microbiology on 31 July 2023 by Antunes, M. D. S. M., Sugiyama, F. H. C., et al.
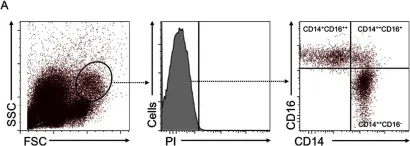
Trained immunity is the enhanced innate immune response resulting from exposure to pathogens or vaccines against an unrelated pathogen stimulus. Certain vaccines induce a memory like response in monocytes and NK cells, leading to modulation in cytokine production, metabolic changes, and modifications in histone patterns. Here, we hypothesized that vaccination against SARS-CoV-2 could induce the training of monocytes in addition to stimulating the adaptive immune response.
Therefore, we aimed to investigate the immunophenotyping, cytokine and metabolic profile of monocytes from individuals who were completely immunized with two doses of inactivated COVID-19 vaccine or non-replicating viral vector vaccine. Subsequently, we investigated the epigenetic mechanisms underlying monocyte immune training. As a model of inflammatorychallenge, to understand if the monocytes were trained by vaccination and how they were trained, cells were stimulated in vitro with the endotoxin LPS, an unrelated stimulus that would provoke the effects of training.
When challenged in vitro, monocytes from vaccinated individuals produced less TNF-α and those who received inactivated vaccine produced less IL-6, whereas vaccination with non-replicating viral vector vaccine induced more IL-10. Inactivated vaccine increased classical monocyte frequency, and both groups showed higher CD163 expression, a hallmark of trained immunity. We observed increased expression of genes involved in glycolysis and reduced IRG1 expression in vaccinated subjects, a gene associated with the tolerance phenotype in monocytes. We observed that both vaccines reduced the chromatin accessibility of genes associated with the inflammatory response, the inactivated COVID-19 vaccine trained monocytes to a regulatory phenotype mediated by histone modifications in the IL6 and IL10 genes, while the non-replicating viral vector COVID-19 vaccine trained monocytes to a regulatory phenotype, mediated by histone modifications in the IL6, IL10, TNF, and CCL2 genes.
Our findings support the recognized importance of adopting vaccination against SARS CoV-2, which has been shown to be effective in enhancing the adaptive immune response against the virus and reducing mortality and morbidity rates. Here, we provide evidence that vaccination also modulates the innate immune response by controlling the detrimental inflammatory response to unrelated pathogen stimulation.
Copyright © 2023 Antunes, Sugiyama, Gravina, Castro, Mercado, Lima, Fontanari and Frantz.
-
COVID-19
-
Genetics
-
Immunology and Microbiology
In Front Immunol on 30 October 2023 by Bazargan, S., Bunch, B., et al.
Fig.1.A

-
FC/FACS
-
Homo sapiens (Human)
Collected and cropped from Front Immunol by CiteAb, provided under a CC-BY license
Image 1 of 2
In Sci Rep on 19 December 2016 by Wildgruber, M., Aschenbrenner, T., et al.
Fig.1.A

-
FC/FACS
-
Collected and cropped from Sci Rep by CiteAb, provided under a CC-BY license
Image 1 of 2