Human rotavirus (HRV) is the causative agent of severe dehydrating diarrhea in children under the age of five, resulting in up to 215,000 deaths each year. These deaths almost exclusively occur in low- and middle-income countries where vaccine efficacy is the lowest due to chronic malnutrition, gut dysbiosis, and concurrent enteric viral infection. Parenteral vaccines for HRV are particularly attractive as they avoid many of the concerns associated with currently used live oral vaccines. In this study, a two-dose intramuscular (IM) regimen of the trivalent, nanoparticle-based, nonreplicating HRV vaccine (trivalent S60-VP8*), utilizing the shell (S) domain of the capsid of norovirus as an HRV VP8* antigen display platform, was evaluated for immunogenicity and protective efficacy against P[6] and P[8] HRV using gnotobiotic pig models. A prime-boost strategy using one dose of the oral Rotarix® vaccine, followed by one dose of the IM trivalent nanoparticle vaccine was also evaluated. Both regimens were highly immunogenic in inducing serum virus neutralizing, IgG, and IgA antibodies. The two vaccine regimens failed to confer significant protection against diarrhea; however, the prime-boost regimen significantly shortened the duration of virus shedding in pigs challenged orally with the virulent Wa (G1P[8]) HRV and significantly shortened the mean duration of virus shedding, mean peak titer, and area under the curve of virus shedding after challenge with Arg (G4P[6]) HRV. Prime-boost-vaccinated pigs challenged with P[8] HRV had significantly higher P[8]-specific IgG antibody-secreting cells (ASCs) in the spleen post-challenge. Prime-boost-vaccinated pigs challenged with P[6] HRV had significantly higher numbers of P[6]- and P[8]-specific IgG ASCs in the ileum, as well as significantly higher numbers of P[8]-specific IgA ASCs in the spleen post-challenge. These results suggest the promise of and warrant further investigation into the oral priming and parenteral boosting strategy for future HRV vaccines.
Product Citations: 58
In Vaccines on 2 May 2023 by Hensley, C., Nyblade, C., et al.
-
FC/FACS
-
Immunology and Microbiology
-
Veterinary Research
Forced enhancer-promoter rewiring to alter gene expression in animal models.
In Molecular Therapy. Nucleic Acids on 14 March 2023 by Peslak, S. A., Demirci, S., et al.
Transcriptional enhancers can be in physical proximity of their target genes via chromatin looping. The enhancer at the β-globin locus (locus control region [LCR]) contacts the fetal-type (HBG) and adult-type (HBB) β-globin genes during corresponding developmental stages. We have demonstrated previously that forcing proximity between the LCR and HBG genes in cultured adult-stage erythroid cells can activate HBG transcription. Activation of HBG expression in erythroid cells is of benefit to patients with sickle cell disease. Here, using the β-globin locus as a model, we provide proof of concept at the organismal level that forced enhancer rewiring might present a strategy to alter gene expression for therapeutic purposes. Hematopoietic stem and progenitor cells (HSPCs) from mice bearing human β-globin genes were transduced with lentiviral vectors expressing a synthetic transcription factor (ZF-Ldb1) that fosters LCR-HBG contacts. When engrafted into host animals, HSPCs gave rise to adult-type erythroid cells with elevated HBG expression. Vectors containing ZF-Ldb1 were optimized for activity in cultured human and rhesus macaque erythroid cells. Upon transplantation into rhesus macaques, erythroid cells from HSPCs expressing ZF-Ldb1 displayed elevated HBG production. These findings in two animal models suggest that forced redirection of gene-regulatory elements may be used to alter gene expression to treat disease.
© 2023 The Author(s).
-
FC/FACS
In Science Advances on 25 November 2022 by Sohn, H. S., Choi, J. W., et al.
Local inflammation in the joint is considered to contribute to osteoarthritis (OA) progression. Here, we describe an immunomodulating nanoparticle for OA treatment. Intradermal injection of lipid nanoparticles (LNPs) loaded with type II collagen (Col II) and rapamycin (LNP-Col II-R) into OA mice effectively induced Col II-specific anti-inflammatory regulatory T cells, substantially increased anti-inflammatory cytokine expression, and reduced inflammatory immune cells and proinflammatory cytokine expression in the joints. Consequently, LNP-Col II-R injection inhibited chondrocyte apoptosis and cartilage matrix degradation and relieved pain, while injection of LNPs loaded with a control peptide and rapamycin did not induce these events. Adoptive transfer of CD4+CD25+ T cells isolated from LNP-Col II-R-injected mice suggested that Tregs induced by LNP-Col II-R injection were likely responsible for the therapeutic effects. Collectively, this study suggests nanoparticle-mediated immunomodulation in the joint as a simple and effective treatment for OA.
-
IHC
-
Mus musculus (House mouse)
-
Immunology and Microbiology
In Journal of Dairy Science on 1 November 2022 by Widmer, J., Descloux, L., et al.
Somatic cell count (SCC) in milk is an essential indicator for defining and managing udder health. However, analyzing differential SCC (dSCC) can be helpful in determining the type or evolution stage of mastitis. A high abundance of polymorphonuclear cells (PMN) is associated with acute mastitis; however, the status of a chronic disease is less well characterized. A method capable of analyzing SCC and dSCC can prove to be a helpful tool for monitoring the status of evolution of mastitis disease in a better way. Therefore, a new direct-flow cytometry method was developed to count and differentiate somatic cells in milk without the steps of centrifugation or washing, avoiding variabilities that occur due to enrichment or loss of specific cell types. In this new method, SCC is analyzed using the method of DNA staining with Hoechst stain, whereas dSCC are analyzed using specific antibodies targeting 2 main cell types associated with mastitis: PMN cells and antigen-presenting cells, which are associated with innate and adaptive immunity. Equivalent SCC values were obtained between the new method and the routine ISO 13366-2 method in a comparison of 240 raw milk samples. Furthermore, dSCC results were confirmed by microscopy after May-Gründwald-Giemsa staining in 165 quarter milk samples from healthy and diseased cows. The method was verified with fluorescence microscopy on the 2 targeted cell types and in raw milk samples. The newly developed method is independent of any instrument and can be further designed to differentiate other cell types and animal species by selecting appropriate antibodies.
The Authors. Published by Elsevier Inc. and Fass Inc. on behalf of the American Dairy Science Association®. This is an open access article under the CC BY license (http://creativecommons.org/licenses/by/4.0/).
-
ICC-IF
In Nature Communications on 28 June 2022 by Vincendeau, E., Wei, W., et al.
SHLD1 is part of the Shieldin (SHLD) complex, which acts downstream of 53BP1 to counteract DNA double-strand break (DSB) end resection and promote DNA repair via non-homologous end-joining (NHEJ). While 53BP1 is essential for immunoglobulin heavy chain class switch recombination (CSR), long-range V(D)J recombination and repair of RAG-induced DSBs in XLF-deficient cells, the function of SHLD during these processes remains elusive. Here we report that SHLD1 is dispensable for lymphocyte development and RAG-mediated V(D)J recombination, even in the absence of XLF. By contrast, SHLD1 is essential for restricting resection at AID-induced DSB ends in both NHEJ-proficient and NHEJ-deficient B cells, providing an end-protection mechanism that permits productive CSR by NHEJ and alternative end-joining. Finally, we show that this SHLD1 function is required for orientation-specific joining of AID-initiated DSBs. Our data thus suggest that 53BP1 promotes V(D)J recombination and CSR through two distinct mechanisms: SHLD-independent synapsis of V(D)J segments and switch regions within chromatin, and SHLD-dependent protection of AID-DSB ends against resection.
© 2022. The Author(s).
-
FC/FACS
-
Mus musculus (House mouse)
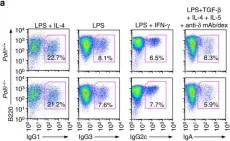
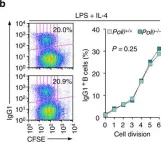
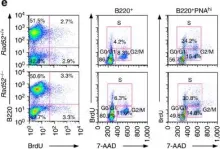
In Nat Commun on 8 February 2017 by Zan, H., Tat, C., et al.
Fig.4.C,D,E

-
FC/FACS
-
Mus musculus (House mouse)
Collected and cropped from Nat Commun by CiteAb, provided under a CC-BY license
Image 1 of 8
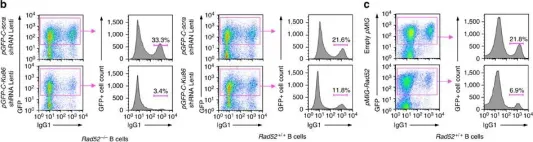
In Nat Commun on 8 February 2017 by Zan, H., Tat, C., et al.
Fig.3.A

-
FC/FACS
-
Mus musculus (House mouse)
Collected and cropped from Nat Commun by CiteAb, provided under a CC-BY license
Image 1 of 8
In Nat Commun on 8 February 2017 by Zan, H., Tat, C., et al.
Fig.3.E

-
FC/FACS
-
Mus musculus (House mouse)
Collected and cropped from Nat Commun by CiteAb, provided under a CC-BY license
Image 1 of 8
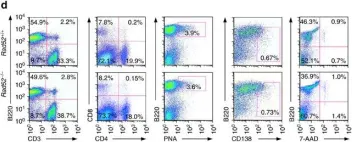
In Nat Commun on 8 February 2017 by Zan, H., Tat, C., et al.
Fig.3.B

-
FC/FACS
-
Mus musculus (House mouse)
Collected and cropped from Nat Commun by CiteAb, provided under a CC-BY license
Image 1 of 8
In Nat Commun on 8 February 2017 by Zan, H., Tat, C., et al.
Fig.8.B,C

-
FC/FACS
-
Mus musculus (House mouse)
Collected and cropped from Nat Commun by CiteAb, provided under a CC-BY license
Image 1 of 8
In Nat Commun on 8 February 2017 by Zan, H., Tat, C., et al.
Fig.4.C

-
FC/FACS
-
Mus musculus (House mouse)
Collected and cropped from Nat Commun by CiteAb, provided under a CC-BY license
Image 1 of 8
In Nat Commun on 8 February 2017 by Zan, H., Tat, C., et al.
Fig.4.D

-
FC/FACS
-
Mus musculus (House mouse)
Collected and cropped from Nat Commun by CiteAb, provided under a CC-BY license
Image 1 of 8
In Nat Commun on 8 February 2017 by Zan, H., Tat, C., et al.
Fig.4.E

-
FC/FACS
-
Mus musculus (House mouse)
Collected and cropped from Nat Commun by CiteAb, provided under a CC-BY license
Image 1 of 8