Cancer-associated fibroblasts (CAFs) are major constituents of the tumor microenvironment (TME) and have been associated with chemotherapeutic failure via different mechanisms. However, the CAFs inhibit chemotherapy mechanism in lung adenocarcinoma (LUAD) remains undetermined.
Fibroblasts were isolated from tumor and normal lung tissues from patients with poorly differentiated LUAD (pCAFs), moderately differentiated LUAD (mCAFs), and normal fibroblasts (NFs). Then, the influence of these fibroblasts on carboplatin's cytotoxic effects on LUAD cell lines A549 and NCI-H1299 was assessed by measuring their IC50 values. Furthermore, CXCL12 secretion and its role in chemotherapeutics were also evaluated.
The data revealed that pCAFs significantly inhibited apoptosis in LUAD cells and increased carboplatin IC50 values. Furthermore, pCAFs secreted higher CXCL12 content than mCAFs and NFs. Moreover, in pCAFs, CXCL12 silencing enhanced carboplatin's cytotoxic effects, while NFs overexpressing CXCL12 inhibited carboplatin's efficacy. Mechanistically, pCAFs promote the secretion of CXCL12 by activating the NF-κB pathway, and CXCL12 binds to CXCR4 on LUAD cells, thereby promoting carboplatin resistance. Moreover, in the xenograft models, pCAFs were found to reduce carboplatin's cytotoxicity by CXCL12 secretion. Moreover, the analysis of the LUAD patient's tumor and peripheral blood sample indicated a correlation between lower differentiation and higher CXCL12 expression levels.
This study revealed that LUAD-derived CAFs activate the NF-κB axis to secrete CXCL12, thereby weakening the carboplatin's killing effect on LUAD. Furthermore, poorly differentiated LUAD secreted more CXCL12. These findings indicate a novel strategy to enhance carboplatin's chemotherapeutic potential against LUAD.
© 2025. The Author(s).
Product Citations: 71
CAFs mediate carboplatin resistance in LUAD via CXCL12 secretion regulated by NF-κB activation.
In Cellular Oncology (Dordrecht) on 1 December 2025 by Li, L., Zhu, X., et al.
Preprint on BioRxiv : the Preprint Server for Biology on 24 October 2025 by Mordelt, A., Schuurmans, I. M. E., et al.
Microglia-neuron interactions play a central role in a variety of central nervous system disorders. Technologies using human induced pluripotent stem cells (hiPSCs) have been developed to model human brain cells with the goal to understand their function. To effectively study neuro-immune crosstalk and investigate microglial contributions to neuronal network development and function, both microglia and neurons should co-mature allowing for long-term interactions throughout their differentiation. Here, we present a co-maturation protocol that robustly generates glutamatergic neuronal networks containing human iPSC-derived microglia. We validated the long-term co-cultures using single-cell transcriptomics, imaging and neuronal activity readouts. We show that astrocytes were required for long-term survival of microglia and for their integration into neuronal networks. Our co-maturation approach induced the typical ramified microglia morphology and characteristic microglia-neuron interactions. Homeostatic markers like P2RY12 and TMEM119 and neuronal remodeling associated genes were upregulated compared to microglia monocultures, highlighting the necessity of the environment to generate and maintain the context-dependent microglia signature in vitro. In this manuscript, we include the full optimization process of our co-maturation approach, a comprehensive description of the protocol, practical guidelines and troubleshooting tips. Our co-maturation model provides a powerful tool to assess the role of human microglia in modulating neuronal function and development in health and disease. Highlights ptimized human iPSC differentiation protocol that allows for co-maturation of microglia and Neurogenin2 -induced neuronal networks. are required for long-term survival and integration of microglia into neuronal networks. Co-maturation approach enables characteristic neuron-microglia interactions and induces signature morphology, transcriptome and proteins of human microglia.
-
FC/FACS
-
Neuroscience
-
Stem Cells and Developmental Biology
In Cell Division on 2 October 2025 by Zhang, X., Zhang, S., et al.
This study investigates how Fibroblast Growth Factor 4 (FGF4) drives triple-negative breast cancer (TNBC) progression by modulating macrophage polarization through the IL6/STAT3 signaling axis, with a focus on immune suppression and tumor microenvironment remodeling.
TNBC transcriptomic datasets from The Cancer Genome Atlas (TCGA) and Gene Expression Omnibus (GEO) were analyzed to identify FGF4-associated pathways using differential gene expression analysis, Weighted Gene Co-expression Network Analysis (WGCNA), and immune infiltration profiling via Cell-type Identification By Estimating Relative Subsets Of RNA Transcripts (CIBERSORT). Functional annotations (GO/KEGG) highlighted IL6/STAT3 as a key pathway. In vitro models with FGF4-overexpressing or knockdown TNBC cells were co-cultured with macrophages to assess IL6/STAT3 activation, M2 polarization markers (CD206, Arg1), and cytokine secretion (ELISA). Tumor cell behaviors (proliferation, migration, invasion) were quantified. In vivo orthotopic TNBC models in mice evaluated FGF4's impact on tumor growth, immune cell infiltration (flow cytometry), and STAT3 activity (Western blot).
FGF4 was upregulated in TNBC and strongly correlated with M2 macrophage infiltration. In vitro, FGF4 activated IL6/STAT3 signaling, inducing macrophage polarization to an M2 phenotype with elevated IL-10/TGF-βsecretion and suppression of T cell proliferation. Conditioned media from M2 macrophages enhanced TNBC cell aggressiveness. In vivo, FGF4-overexpressing tumors showed higher weight and increased M2 markers, whereas FGF4 knockdown reduced tumor volume and enhanced CD8+ T cell infiltration.
FGF4 promotes TNBC progression by activating IL6/STAT3 to reprogram macrophages into immune-suppressive M2 effectors, fostering a tumor-permissive microenvironment. Targeting FGF4 may disrupt this crosstalk, offering a novel immunotherapeutic strategy for TNBC.
© 2025. The Author(s).
-
FC/FACS
-
Cancer Research
-
Immunology and Microbiology
In Acta Neuropathologica Communications on 28 June 2025 by Alrefai, H., Lin, B., et al.
Tumor-associated macrophages (TAMs) are the most abundant non-cancerous cell type in glioblastoma (GBM) and heavily influence GBM biology, contributing to tumor progression, therapeutic resistance, immune evasion, and neovascularization. Current in vitro models that utilize IL-4/IL-13 stimulation fail to capture the transcriptional and functional heterogeneity of TAMs observed in vivo. In this study, we utilize a serum-free indirect co-culture model with patient-derived xenolines to polarize primary human macrophages and characterize their molecular and functional phenotypes. We demonstrate that xenoline-polarized macrophages diverge from classical M1/M2 states and instead adopt transcriptional signatures reflective of TAM subsets identified from patients. Notably, macrophages polarized with the radiation-therapy selected xenoline, JX14P-RT, exhibited gene expression patterns enriched for interferon response and hypoxia, mirroring recurrent GBM samples. In contrast, JX14P TAMs showed enrichment in phagocytic gene sets. Functional validation of these phenotypes revealed discrepancies between the transcriptionally predicted and observed phenotypes, emphasizing the importance of integrating phenotypic validation in sequencing studies. Altogether, our findings establish xenoline-polarized macrophages as a useful alternative to traditional models that can be used to study immune-interactions in vitro.
© 2025. The Author(s).
-
ICC-IF
-
Homo sapiens (Human)
-
Cancer Research
Preprint on Research Square on 9 May 2025 by Alrefai, H., Lin, B., et al.
Abstract Tumor-associated macrophages (TAMs) are the most abundant non-cancerous cell type in glioblastoma (GBM) and heavily influence GBM biology, contributing to tumor progression, therapeutic resistance, immune evasion, and neovascularization. Current in vitro models that utilize IL-4/IL-13 stimulation fail to capture the transcriptional and functional heterogeneity of TAMs observed in vivo. In this study, we utilize a serum-free indirect co-culture model with patient-derived xenolines to polarize primary human macrophages and characterize their molecular and functional phenotypes. We demonstrate that xenoline-polarized macrophages diverge from classical M1/M2 states and instead adopt transcriptional signatures reflective of TAM subsets identified from patients. Notably, macrophages polarized with the radiation-therapy selected xenoline, JX14P-RT, exhibited gene expression patterns enriched for interferon response and hypoxia, mirroring recurrent GBM samples. In contrast, JX14P TAMs showed enrichment in phagocytic gene sets. Functional validation of these phenotypes revealed discrepancies between the transcriptionally predicted and observed phenotypes, emphasizing the importance of integrating phenotypic validation in sequencing studies. Altogether, our findings establish xenoline-polarized macrophages as a more physiologically relevant alternative to traditional models, offering a useful model for studying tumor-immune interaction in vitro.
-
ICC-IF
-
Cancer Research
In Sci Rep on 21 July 2021 by Durlanik, S., Fundel-Clemens, K., et al.
Fig.2.D
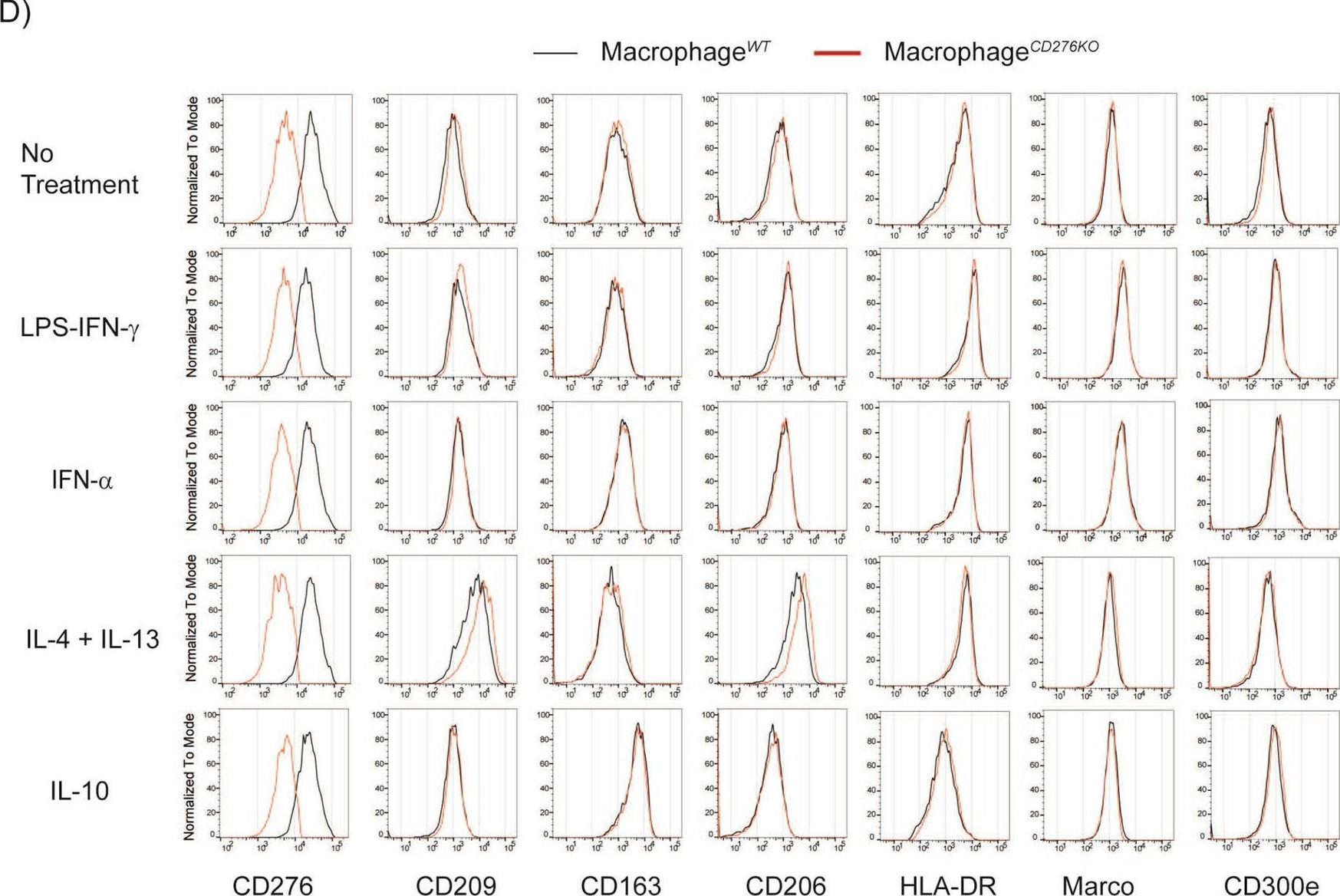
-
FC/FACS
-
Homo sapiens (Human)
Collected and cropped from Scientific Reports by CiteAb, provided under a CC-BY license
Image 1 of 1
