Abstract Therapeutic strategies for peritoneal metastasis in solid tumors are urgently needed in the clinic. Programming chimeric antigen receptor macrophages (CAR-Ms) in situ offers opportunities for an unmet demand. However, potential intracellular domains (ICDs) for CAR design and their antitumor mechanisms for macrophage empowerment remain to be explored systematically. By developing a targeted mRNA-LNP delivery system for macrophages, we have investigated 36 CAR combinations to determine the impact of CAR-Ms on immune regulation in vitro and in vivo. In two solid tumor mouse models, intraperitoneal programming of CAR-Ms was shown to elicit robust adaptive immune activation and significantly synergize with PD-1/L1 therapy. Single-cell RNA sequencing (scRNA-seq) analysis revealed that CAR-Ms could reshape the immunosuppressive tumor microenvironment (TME) and boost the TCF1+PD-1+ progenitor-exhausted CD8+ T cells (Tpex) population. Meanwhile, we found that tailored CAR-M with CD3ζ/TLR4 ICDs could favorably maintain proinflammatory phenotype and simultaneously upregulate MHC I and PD-L1 expression by perturbing NF-κB pathways. Moreover, the synergism between macrophage PD-L1 knockdown and CAR-M therapy highlighted the need to block the PD-1/L1 axis in antigen cross-presentation. In short, we developed an mRNA-LNP delivery system for intraperitoneal programming of tailored CAR-Ms in vivo and broadened understanding of both regulatory and feedback mechanisms for CAR-M therapies against solid tumors.
Product Citations: 204
Intraperitoneal programming of tailored CAR macrophages via mRNA-LNP to boost cancer immunotherapy
Preprint on Research Square on 7 March 2025 by Xie, S., Gu, K., et al.
-
Cancer Research
-
Genetics
-
Immunology and Microbiology
In Molecular Medicine on 31 December 2024 by Chen, H., Li, Y., et al.
Partial stereotactic body radiation therapy (SBRT) targeting hypoxic regions of large tumors (SBRT-PATHY) has been shown to enhance the efficacy of tumor radiotherapy by harnessing the radiation-induced immune response. This approach suggests that reducing the irradiation target volume not only achieves effective anti-tumor effects but also minimizes damage to surrounding normal tissues. In this study, we evaluated the antitumor efficacy of reduced-tumour-area radiotherapy (RTRT) , and explored the relationship between tumor control and immune preservation and the molecular mechanisms underlying of them.
In mouse breast cancer models, we compared the anti-tumor effects of RTRT and conventional radiotherapy (CNRT) by assessing tumor growth, metastasis, and survival rates. Additionally, we evaluated the peritumoral tissue damage and the immune microenvironment. The maturation of dendritic cells (DCs) and DNA damage induced by irradiated tumor cells were also assessed in vitro.
In pre-clinical models, both RTRT and CNRT significantly inhibited primary tumor growth when compared to non-irradiated controls, with no significant difference between RTRT and CNRT. However, RTRT significantly extended survival times in mice, and increased the likelihood of inducing abscopal effects, thereby providing potential for better control of distant metastases. Further investigations revealed that the enhanced efficacy of RTRT may be attributed to the preservation of lymphocytes within the peritumoral tissue, as well as reduced damage to the surrounding skin and circulating lymphocytes. In vitro assays demonstrated that RTRT induced DNA damage and dsDNA in tumor cells, activating the cGAS-STING pathway. RTRT also triggered the release of damage-associated molecular patterns (DAMPs), which synergistically amplified the anti-tumor immune response.
Our findings suggested that appropriately narrowing the irradiation target volume effectively killed tumor cells while reducing damage to surrounding tissues, and preserving peritumoral lymphocytes. This approach improved the safety of radiotherapy while maintaining its efficacy in tumor control and provided an opportunity for combining high-dose radiotherapy with immunotherapy.
© 2024. The Author(s).
-
Mus musculus (House mouse)
-
Biochemistry and Molecular biology
-
Cancer Research
-
Genetics
In Nature Communications on 14 November 2024 by Vijayan, A., Vogels, R., et al.
Newly approved subunit and mRNA vaccines for respiratory syncytial virus (RSV) demonstrate effectiveness in preventing severe disease, with protection exceeding 80% primarily through the generation of antibodies. An alternative vaccine platform called self-amplifying RNA (saRNA) holds promise in eliciting humoral and cellular immune responses. We evaluate the immunogenicity of a lipid nanoparticle (LNP)-formulated saRNA vaccine called SMARRT.RSV.preF, encoding a stabilized form of the RSV fusion protein, in female mice and in non-human primates (NHPs) that are either RSV-naïve or previously infected. Intramuscular vaccination with SMARRT.RSV.preF vaccine induces RSV neutralizing antibodies and cellular responses in naïve mice and NHPs. Importantly, a single dose of the vaccine in RSV pre-exposed NHPs elicits a dose-dependent anamnestic humoral immune response comparable to a subunit RSV preF vaccine. Notably, SMARRT.RSV.preF immunization significantly increases polyfunctional RSV.F specific memory CD4+ and CD8+ T-cells compared to RSV.preF protein vaccine. Twenty-four hours post immunization with SMARRT.RSV.preF, there is a dose-dependent increase in the systemic levels of inflammatory and chemotactic cytokines associated with the type I interferon response in NHPs, which is not observed with the protein vaccine. We identify a cluster of analytes including IL-15, TNFα, CCL4, and CXCL10, whose levels are significantly correlated with each other after SMARRT.RSV.preF immunization. These findings suggest saRNA vaccines have the potential to be developed as a prophylactic RSV vaccine based on innate, cellular, and humoral immune profiles they elicit.
© 2024. The Author(s).
-
Mus musculus (House mouse)
-
Genetics
-
Immunology and Microbiology
Preprint on BioRxiv : the Preprint Server for Biology on 4 November 2024 by Zhang, D., Xiao, Q., et al.
There continues to be a dearth of competent inhalable mRNA delivery although it holds great potential for addressing a wide variety of refractory diseases. The huge advances seen with parenteral-administered lipid nanoparticle (LNP) have not been translated into nebulized mRNA delivery due to the aggressive nebulization process and insurmountable barriers inherent to respiratory mucosa. Here, we show amphiphilic block copolymers revealed by machine learning (ML) can spontaneously form stabilized nanoparticles (PoLixNano) with the lipids components of LNP and simultaneously impart the PoLixNano with "shield" (shear force-resistant) and "spear" (pulmonary barriers-penetrating abilities) capabilities. We present a ML approach that leverages physicochemical properties and inhaled mRNA transfection profiles of a chemically diverse library of polymeric components to validate the integration of "shield" and "spear" properties as highly predictive indicators of transfection efficiency. This quantitative structure-mRNA transfection prediction (QSMTP) model identifies top-performing amphiphilic-copolymers from more than 10000 candidates and suggests their mucus-penetrating ability outweights the shear force-resistant property in contributing to efficient mRNA transfection. The optimized PoLixNano substantially outperforms the LNP counterpart and mediates up to 1114-times higher levels of mRNA transfection in animal models with negligible toxicities. The PoLixNano promotes overwhelming SARS-CoV-2 antigen-specific sIgA antibody secretion and expansion of TRM cells which collectively confers 100% protection in mice against lethal SARS-CoV-2 challenges and blocks the transmission of Omicron variant between hamsters. PoLixNano also displays versatile therapeutic potential in lung carcinoma and cystic fibrosis models. Our study provides new insights for designing delivery platforms of aerosol-inhaled mRNA therapeutics with clinical translation potential.
-
Genetics
Preprint on BioRxiv : the Preprint Server for Biology on 22 September 2024 by Zhang, J., Li, C., et al.
The development of next-generation mucosal mRNA vaccines is promising but extremely challenging. Major efforts have been focused on optimizing delivery systems, whereas it is still unknown whether the intrinsic quality of IVT mRNA significantly impacts the potency of airway inoculated mRNA vaccines. Here, we systematically demonstrate the mucosal mRNA vaccine requires a higher standard of purification and tailor-designed sequence to fulfil its potency compared to the parenteral route inoculated counterpart. We found double strand RNA (dsRNA) contaminants are prone to trigger innate immunoreaction in the airway that activates the mRNA degradation mechanism, thereby diminishing the mRNA expression and subsequent antigen-specific immune responses. To address these challenges, we developed a strategy that combines optimized untranslated regions (UTRs) screened from endogenous genes of pulmonary cells with affinity chromatography-based purification which removes almost all the dsRNA contaminants. The optimized mRNA administered via the airway route not only demonstrates superior protein expression (30-fold increase) and reduces inflammation in the lung, but also promotes robust immunity comprising significantly elevated systemic, cellular, and mucosal immune responses, which is in stark contrast to intramuscular injected counterpart that displays less pronounced benefits. Our findings offer new insight into the development of mucosal mRNA therapeutics from an overlooked but crucial perspective of optimizing mRNA components.
-
Mus musculus (House mouse)
-
Genetics
-
Immunology and Microbiology
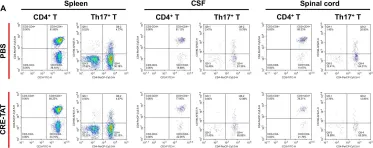
In J Neuroinflammation on 18 February 2022 by Zheng, W., Feng, Y., et al.
Fig.5.A

-
FC/FACS
-
Mus musculus (House mouse)
Collected and cropped from J Neuroinflammation by CiteAb, provided under a CC-BY license
Image 1 of 1