Decellularized scaffolds are promising biomaterials for tissue and organ reconstruction; however, strategies to effectively suppress the host immune responses toward these implants, particularly those without chemical crosslinking, remain warranted. Administration of mesenchymal stem cells is effective against immune-mediated inflammatory disorders. Herein, we investigated the effect of isogeneic abdominal adipose-derived mesenchymal stem/stromal cells (ADMSCs) on xenogeneic biomaterial-induced immunoreactions. Peripheral bronchi from pigs, decellularized using a detergent enzymatic method, were engrafted onto tracheal defects of Brown Norway (BN) rats. BN rats were implanted with native pig bronchi (Xenograft group), decellularized pig bronchi (Decellularized Xenograft), or Decellularized Xenograft and ADMSCs (Decellularized Xenograft+ADMSC group). In the latter group, ADMSCs were injected intravenously immediately post implantation. Harvested graft implants were assessed histologically and immunohistochemically. We found that acute rejections were milder in the Decellularized Xenograft and Decellularized Xenograft+ADMSC groups than in the Xenograft group. Mild inflammatory cell infiltration and reduced collagen deposition were observed in the Decellularized Xenograft+ADMSC group. Additionally, ADMSC administration decreased CD8+ lymphocyte counts but increased CD163+ cell counts. In the Decellularized Xenograft+ADMSC group, serum levels of vascular endothelial growth factor and IL-10 were elevated and tissue deposition of IgM and IgG was low. The significant immunosuppressive effects of ADMSCs illustrate their potential use as immunosuppressive agents for xenogeneic biomaterial-based implants.
Product Citations: 32
In Organogenesis on 31 December 2023 by Hisanaga, M., Tsuchiya, T., et al.
-
Rattus norvegicus (Rat)
-
Immunology and Microbiology
-
Stem Cells and Developmental Biology
-
Veterinary Research
In Cells on 5 September 2022 by Mao, X., Yao, L., et al.
(1) Background: Reconstruction of Achilles tendon defects and prevention of postoperative tendon adhesions were two serious clinical problems. In the treatment of Achilles tendon defects, decellularized matrix materials and mesenchymal stem cells (MSCs) were thought to address both problems. (2) Methods: In vitro, cell adhesion, proliferation, and tenogenic differentiation of tendon-derived stem cells (TDSCs) on small intestinal submucosa (SIS) were evaluated. RAW264.7 was induced by culture medium of TDSCs and TDSCs-SIS scaffold groups. A rat Achilles tendon defect model was used to assess effects on tendon regeneration and antiadhesion in vivo. (3) Results: SIS scaffold facilitated cell adhesion and tenogenic differentiation of TDSCs, while SIS hydrogel coating promoted proliferation of TDSCs. The expression of TGF-β and ARG-1 in the TDSCs-SIS scaffold group were higher than that in the TDSCs group on day 3 and 7. In vivo, the tendon regeneration and antiadhesion capacity of the implanted TDSCs-SIS scaffold was significantly enhanced. The expression of CD163 was significantly highest in the TDSCs-SIS scaffold group; meanwhile, the expression of CD68 decreased more significantly in the TDSCs-SIS scaffold group than the other two groups. (4) Conclusion: This study showed that biologically prepared SIS scaffolds synergistically promote tendon regeneration with TDSCs and achieve antiadhesion through M2 polarization of macrophages.
-
FC/FACS
-
Rattus norvegicus (Rat)
-
Cell Biology
-
Immunology and Microbiology
-
Stem Cells and Developmental Biology
In International Journal of Medical Sciences on 17 April 2021 by Lee, S. S., Wu, Y. C., et al.
Augmentative and reconstructive rhinoplasty surgical procedures use autologous tissue grafts or synthetic grafts to repair the nasal defect and aesthetic reconstruction. Donor site trauma and morbidity are common in autologous grafts. The desperate need for the production of grafted 3D cartilage tissues as rhinoplasty grafts without the adverse effect is the need of the hour. In the present study, we developed a bioactive 3D histotypic construct engineered with the various ratio of adipose-derived stem cells (ADSC) and chondrocytes together with decellularized porcine nasal cartilage graft (dPNCG). We decellularized porcine nasal cartilage using supercritical carbon dioxide (SCCO2) extraction technology. dPNCG was characterized by H&E, DAPI, alcian blue staining, scanning electron microscopy and residual DNA content, which demonstrated complete decellularization. 3D histotypic constructs were engineered using dPNCG, rat ADSC and chondrocytes with different percentage of cells and cultured for 21 days. dPNCG together with 100% chondrocytes produced a solid mass of 3D histotypic cartilage with significant production of glycosaminoglycans. H&E and alcian blue staining showed an intact mass, with cartilage granules bound to one another by extracellular matrix and proteoglycan, to form a 3D structure. Besides, the expression of chondrogenic markers, type II collagen, aggrecan and SOX-9 were elevated indicating chondrocytes cultured on dPNCG substrate facilitates the synthesis of type II collagen along with extracellular matrix to produce 3D histotypic cartilage. To conclude, dPNCG is an excellent substrate scaffold that might offer a suitable environment for chondrocytes to produce 3D histotypic cartilage. This engineered 3D construct might serve as a promising future candidate for cartilage tissue engineering in rhinoplasty.
© The author(s).
-
FC/FACS
-
Rattus norvegicus (Rat)
-
Veterinary Research
In Cell Cycle on 1 January 2021 by Zhu, J., Fu, Q., et al.
This study explored the role of MEG3 in the cartilage differentiation of bone marrow mesenchymal stem cells (BMSCs). We investigated the effects of over-expression and knockdown of MEG3 on cell viability, cell differentiation, and the expressions of MEG3, miR-129-5p, COL2, chondrocyte differentiation-related genes (sry-type high-mobility-group box 9 (SOX9), SOX5, Aggrecan, silent information regulator 1 (SIRT1), and Cartilage oligomeric matrix protein (COMP)). The targeting relationship between MEG3 and miR-129-5p and the target gene of miR-129-5p was confirmed through Starbase, TargetScan and luciferase experiments. Finally, a series of rescue experiments were conducted to study the regulatory effects of MEG3 and miR-129-5p. BMSCs were identified as CD29+ and CD44+ positive, and their differentiation was time-dependent. As BMSCs differentiated, MEG3 expression was up-regulated, but miR-129-5p was down-regulated. Over-expressed MEG3 promoted the viability and differentiation of BMSCs, up-regulated the expressions of COL2 and chondrocyte differentiation-related genes, and inhibited miR-129-5p. Runt-related transcription factor 1 (RUNX1) was negatively regulated as a target gene of miR-129-5p. Results of rescue experiments showed that the inhibitory effect of miR-129-5p mimic on BMSCs could be partially reversed by MEG3. Over-expression of MEG3 regulated the miR-129-5p/RUNX1 axis to promote the differentiation of BMSCs into chondrocytes. This study provides a reliable basis for the application of lncRNA in articular cartilage injury.
-
FC/FACS
-
Cell Biology
-
Stem Cells and Developmental Biology
In Aging (Albany NY) on 3 December 2020 by Luo, Y., Wang, B., et al.
Content and aims: Ginsenoside RG1 (RG1) is thought to enhance proliferation and differentiation of stem cell, however, its role on paracrine efficacy of stem cell remains unclear. Here we examined if and how RG1 enhances the paracrine effects of bone marrow-derived mesenchymal stem cells (BM-MSCs) on radiation induced intestinal injury (RIII).
Irradiated rats randomly received intraperitoneal injection of conditioned medium (CM) derived from non-activated BM-MSCs (MSC-CM) or BM-MSCs pre-activated by RG-1 (RG1-MSC-CM). Intestinal samples were collected, followed by the evaluation of histological and functional change, apoptosis, proliferation, inflammation, angiogenesis and stem cell regeneration. The effects of heme oxygenase-1 (HO-1) were investigated using HO-1 inhibitor or siRNA.
RG1 enhanced the paracrine efficacy of BM-MSCs partially through upregulation of HO-1. RG1-MSC-CM rather than MSC-CM significantly improved the survival and intestinal damage of irradiated rats via improvement of intestinal proliferation/apoptosis, inflammation, angiogenesis and stem cell regeneration in a HO-1 dependent mechanism. The mechanism for the superior paracrine efficacy of RG1-MSC-CM is related to a higher release of two pivotal cytokines VEGF and IL-6.
Our study revealed that RG1 enhances paracrine effects of BM-MSCs on RIII, providing a novel method for maximizing the paracrine potential of MSCs.
-
FC/FACS
-
Rattus norvegicus (Rat)
-
Endocrinology and Physiology
-
Stem Cells and Developmental Biology
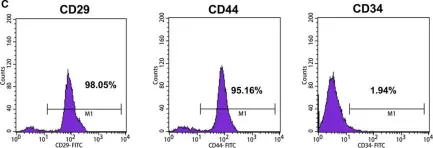
In J Cell Mol Med on 1 October 2018 by Sun, J., Zhao, F., et al.
Fig.1.C

-
FC/FACS
-
Rattus norvegicus (Rat)
Collected and cropped from J Cell Mol Med by CiteAb, provided under a CC-BY license
Image 1 of 2
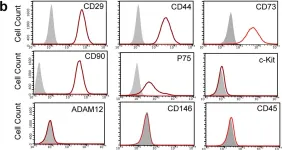
In Sci Rep on 10 January 2017 by Wang, D., Wang, A., et al.
Fig.3.B

-
FC/FACS
-
Rattus norvegicus (Rat)
Collected and cropped from Sci Rep by CiteAb, provided under a CC-BY license
Image 1 of 2