Autophagy, a cytoprotective mechanism in intestinal epithelial cells, plays a crucial role in maintaining intestinal homeostasis. Beyond its cell-autonomous effects, the significance of autophagy in these cells is increasingly acknowledged in the dynamic interplay between the microbiota and the immune response. In the context of colon cancer, intestinal epithelium disruption of autophagy has been identified as a critical factor influencing tumor development. This disruption modulates the composition of the gut microbiota, eliciting an anti-tumoral immune response. Here, we report that Atg7 deficiency in intestinal epithelial cells shapes the intestinal microbiota leading to an associated limitation of colitis induced by Citrobacter rodentium infection. Mice with an inducible, intestinal epithelial-cell-specific deletion of the autophagy gene, Atg7, exhibited enhanced clearance of C. rodentium, mitigated hyperplasia, and reduced pathogen-induced goblet cell loss. This protective effect is linked to a higher proportion of neutrophils and phagocytic cells in the early phase of infection. At later stages, it is associated with the downregulation of pro-inflammatory pathways and an increase in Th17 and Treg responses-immune responses known for their protective roles against C. rodentium infection, modulated by specific gut microbiota. Fecal microbiota transplantation and antibiotic treatment approaches revealed that the Atg7-deficiency-shapped microbiota, especially Gram-positive bacteria, playing a central role in driving resistance to C. rodentium infection. In summary, our findings highlight that inhibiting autophagy in intestinal epithelial cells contributes to maintaining homeostasis and preventing detrimental intestinal inflammation through microbiota-mediated colonization resistance against C. rodentium. This underscores the central role played by autophagy in shaping the microbiota in promoting immune-mediated resistance against enteropathogens.
© 2025. The Author(s).
Product Citations: 129
Inhibition of Atg7 in intestinal epithelial cells drives resistance against Citrobacter rodentium.
In Cell Death & Disease on 19 February 2025 by Cune, D., Pitasi, C. L., et al.
-
FC/FACS
-
Mus musculus (House mouse)
-
Cell Biology
In EBioMedicine on 1 February 2025 by Papadopoulos, S., Hardy, D., et al.
Leptospirosis is a globally neglected re-emerging zoonosis affecting all mammals, albeit with variable outcomes. Humans are susceptible to leptospirosis; infection with Leptospira interrogans species can cause severe disease in humans, with multi-organ failure, mainly affecting kidney, lung and liver function, leading to death in 10% of cases. Mice and rats are more resistant to acute disease and can carry leptospires asymptomatically in the kidneys and act as reservoirs, shedding leptospires into the environment. The incidence of leptospirosis is higher in tropical countries, and countries with poor sanitation, where heavy rainfall and flooding favour infection. Diagnosis of leptospirosis is difficult because of the many different serovars and the variety of clinical symptoms that can be confused with viral infections. The physiopathology is poorly understood, and leptospirosis is often regarded as an inflammatory disease, like sepsis.
To investigate the causes of death in lethal leptospirosis, we compared intraperitoneal infection of male and female C57BL6/J mice with 108Leptospira of two strains of pathogenic L. interrogans. One strain, L. interrogans Manilae L495, killed the mice 4 days after infection, whereas the other strain, L. interrogans Icterohaemorrhagiae Verdun, did not induce any major symptoms in the mice. On day 3 post infection, the mice were humanely euthanised and blood and organs were collected. Bacterial load, biochemical parameters, cytokine production and leucocyte population were assessed by qPCR, ELISA, cytometry and immunohistochemistry.
Neither lung, liver, pancreas or kidney damage nor massive necroptosis or cytokine storm could explain the lethality. Although we did not find pro-inflammatory cytokines, we did find elevated levels of the anti-inflammatory cytokine IL-10 and the chemokine RANTES in the serum and organs of Leptospira-infected mice. In contrast, severe leptospirosis was associated with neutrophilia and vascular permeability, unexpectedly due to neutrophils and not only due to Leptospira infection. Strikingly, the main cause of death was myocarditis, an overlooked complication of human leptospirosis.
Despite clinical similarities between bacterial sepsis and leptospirosis, striking differences were observed, in particular a lack of cytokine storm in acute leptospirosis. The fact that IL-10 was increased in infected mice may explain the lack of pro-inflammatory cytokines, emphasising the covert nature of Leptospira infections. Neutrophilia is a hallmark of human leptospirosis. Our findings confirm the ineffective control of infection by neutrophils and highlight their deleterious role in vascular permeability, previously only attributed to the ability of leptospires to damage and cross endothelial junctions. Finally, the identification of death due to myocarditis rather than kidney, liver or liver failure may reflect an overlooked but common symptom associated with poor prognosis in human leptospirosis. These features of neutrophilia and myocarditis are also seen in patients, making this mouse model a paradigm for better understanding human leptospirosis and designing new therapeutic strategies.
The Boneca laboratory was supported by the following programmes: Investissement d'Avenir program, Laboratoire d'Excellence "Integrative Biology of Emerging Infectious Diseases" (ANR-10-LABX-62-IBEID) and by R&D grants from Danone and MEIJI. CW received an ICRAD/ANR grant (S-CR23012-ANR 22 ICRD 0004 01). SP received a scholarship by Université Paris Cité (formerly Université Paris V - Descartes) through Doctoral School BioSPC (ED562, BioSPC). SP has additionally received a scholarship "Fin de Thèse de Science" number FDT202404018322 granted by "Fondation pour la Recherche Médicale (FRM)". The funders had no implication in the design, analysis and reporting of the study.
Copyright © 2025 The Authors. Published by Elsevier B.V. All rights reserved.
-
Veterinary Research
In Nucleic Acids Research on 11 January 2025 by Medina-Suárez, D., Han, L., et al.
Recent studies have revealed a structural role for DNA ligase 4 (Lig4) in the maintenance of a repair complex during non-homologous end joining (NHEJ) of DNA double-strand breaks. In cultured cell lines, catalytically inactive Lig4 can partially alleviate the severe DNA repair phenotypes observed in cells lacking Lig4. To study the structural role of Lig4 in vivo, a mouse strain harboring a point mutation to Lig4's catalytic site was generated. In contrast to the ablation of Lig4, catalytically inactive Lig4 mice are born alive. These mice display marked growth retardation and have clear deficits in lymphocyte development. We considered that the milder phenotype results from inactive Lig4 help to recruit another ligase to the repair complex. We next generated a mouse strain deficient for nuclear Lig3. Nuclear Lig3-deficient mice are moderately smaller and have elevated incidences of cerebral ventricle dilation but otherwise appear normal. Strikingly, in experiments crossing these two strains, mice lacking nuclear Lig3 and expressing inactive Lig4 were not obtained. Timed mating revealed that fetuses harboring both mutations underwent resorption, establishing an embryonic lethal genetic interaction. These data suggest that Lig3 is recruited to NHEJ complexes to facilitate end joining in the presence (but not activity) of Lig4.
© The Author(s) 2024. Published by Oxford University Press on behalf of Nucleic Acids Research.
-
Mus musculus (House mouse)
-
Biochemistry and Molecular biology
-
Genetics
Preprint on Research Square on 18 November 2024 by Chen, R., Ding, S., et al.
Abstract Bladder cancer is recognized as one of the most prevalent malignant tumors within the urinary system. The conventional treatment approach for bladder cancer typically involves a combination of surgery, radiotherapy, and chemotherapy. However, the efficacy of current treatment modalities remains suboptimal, prompting ongoing efforts to develop novel and more effective therapeutic strategies to better address the clinical demands of bladder cancer management. In this study, we utilized the orthotopic mouse model to assess the effectiveness of intravesical conventional chemotherapy alone and in combination with immunotherapy for treating bladder cancer. The anti-tumor effect was analyzed by determining bioluminescence imaging (BLI), while histopathological analysis was conducted to evaluate the tumor proliferation and invasion capabilities upon treatment. Additionally, alterations in the immune microenvironment within different treatment methods were studied through flow cytometry for various T-cell markers. BLI and tumor weights analysis revealed that the intravesical route of doxorubicin administration produced better treatment efficacy than the conventional chemotherapy through the intraperitoneal route and combination of doxorubicin and anti-PD-L1 i.p administration. Histopathological analysis and proliferation markers (Ki-67 staining) revealed significant differences across the intravesical, conventional chemotherapy, and immune combination therapy groups. Importantly, intravesical treatment was more effective in reducing tumor cell proliferation compared to the other groups. FACS analysis revealed the route of administration significantly impacted the immune response in the tumor microenvironment. Our results demonstrate that both intravesical and conventional doxorubicin chemotherapy led to a significant decrease in CD8+ T cell expression (p < 0.01), while intravesical treatment exhibited a more pronounced activation of CD8+ T cells, as evidenced by increased CD69 expression. Treg cells also showed moderate reductions in the conventional chemotherapy and immune combination therapy groups. Notably, the intravesical approach activated CD8+ T cells more effectively and reduced the expression of the exhaustion marker PD-1 compared to immune combination therapy. Overall, these findings highlight the potential of intravesical doxorubicin delivery to activate CD8+ T cells and reduce immune exhaustion, enhancing its anti-tumor efficacy. These results suggest that intravesical administration may be a viable treatment option for bladder cancer in clinical settings.
-
Mus musculus (House mouse)
-
Cancer Research
-
Immunology and Microbiology
SETD1B mutations confer apoptosis resistance and BCL2 independence in B cell lymphoma.
In The Journal of Experimental Medicine on 7 October 2024 by Portelinha, A., Wang, S., et al.
The translocation t(14;18) activates BCL2 and is considered the initiating genetic lesion in most follicular lymphomas (FL). Surprisingly, FL patients fail to respond to the BCL2 inhibitor, Venetoclax. We show that mutations and deletions affecting the histone lysine methyltransferase SETD1B (KMT2G) occur in 7% of FLs and 16% of diffuse large B cell lymphomas (DLBCL). Deficiency in SETD1B confers striking resistance to Venetoclax and an experimental MCL-1 inhibitor. SETD1B also acts as a tumor suppressor and cooperates with the loss of KMT2D in lymphoma development in vivo. Consistently, loss of SETD1B in human lymphomas typically coincides with loss of KMT2D. Mechanistically, SETD1B is required for the expression of several proapoptotic BCL2 family proteins. Conversely, inhibitors of the KDM5 histone H3K4 demethylases restore BIM and BIK expression and synergize with Venetoclax in SETD1B-deficient lymphomas. These results establish SETD1B as an epigenetic regulator of cell death and reveal a pharmacological strategy to augment Venetoclax sensitivity in lymphoma.
© 2024 Portelinha et al.
-
FC/FACS
-
Cancer Research
-
Immunology and Microbiology
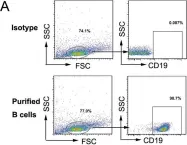
In Cell Death Dis on 16 September 2020 by Li, S., Liu, Q., et al.
Fig.4.A

-
FC/FACS
-
Mus musculus (House mouse)
Collected and cropped from Cell Death Dis by CiteAb, provided under a CC-BY license
Image 1 of 2
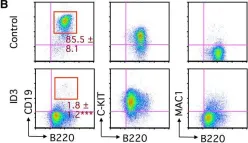
In Stem Cell Reports on 10 November 2015 by Ikawa, T., Masuda, K., et al.
Fig.1.B

-
FC/FACS
-
Mus musculus (House mouse)
Collected and cropped from Stem Cell Reports by CiteAb, provided under a CC-BY license
Image 1 of 2