The mouse synovium can be used to model the sterile inflammation observed in rheumatoid arthritis and osteoarthritis. Here, we present a protocol for inducing arthritis and harvesting and preparing a single-cell suspension from cells of interest from the mouse synovium in steady state or inflammation, with or without intravascular labeling. The prepared single-cell suspension can then be used for downstream analyses, including flow cytometry, fluorescence-activated cell sorting (FACS), both bulk and single-cell RNA sequencing, and other genomic assays. For complete details on the use and execution of this protocol please refer to Montgomery et al.1 and Montgomery et al.2.
Copyright © 2025 The Authors. Published by Elsevier Inc. All rights reserved.
Product Citations: 239
Protocol for preparation of mouse synovium for flow cytometry and RNA-seq.
In STAR Protocols on 13 November 2025 by Montgomery, A. B., Cuda, C. M., et al.
-
Genetics
In Journal of Ginseng Research on 1 November 2025 by Gu, G. R., Kim, H. J., et al.
and design: This research was conducted to investigate the immunomodulatory and anti-inflammatory effects of Korean red ginseng in a low-dose pseudo-type SARS-CoV-2 (PSV) infection model using hACE2 transgenic mice.
K18-hACE2 transgenic mice were used as an experimental model, and three different Korean red ginseng formulations (non-saponin, saponin, and whole Korean red ginseng extract) were administered prior to infection with the virus.
The physiological and immunological effects of the Korean red ginseng formulations were assessed by monitoring lung histopathology and luciferase activity in lung tissue. Flow cytometry was used to analyze the populations of immune cells in both the lungs and spleen, and serum IgM levels were quantified using an enzyme-linked immunosorbent assay.
Low-dose PSV infection induced lung injury and immune cell infiltration in the lung. Administration of Korean red ginseng, particularly the saponin fraction, significantly reduced the excessive activation of interstitial macrophages, NK cells, and cytotoxic T cells. Additionally, Korean red ginseng promoted long-term immune memory by increasing the population of memory B cells and cytotoxic T cells in the spleen. In the treatment groups, IgM production was enhanced upon secondary PSV infection.
Korean red ginseng modulates immune responses and promotes long-term immunity against low-dose PSV infection. Our data also support the potential of Korean red ginseng as a complementary therapeutic strategy for COVID-19 and an enhancer of vaccine efficacy.
© 2025 The Korean Society of Ginseng. Publishing services by Elsevier B.V.
-
FC/FACS
-
Cardiovascular biology
-
COVID-19
-
Immunology and Microbiology
In Science Advances on 24 October 2025 by In Het Panhuis, W., Thiemann, E., et al.
The chimeric cytokine IC7Fc conveys the metabolic signaling properties of the glycoprotein 130 receptor cytokines interleukin-6 and ciliary neurotrophic factor via membrane-bound signaling. IC7Fc was previously shown to slow the progression of type 2 diabetes mellitus, and here, we demonstrate its effect on atherosclerotic development. In APOE*3-Leiden.CETP mice, an atherosclerosis-prone model with a humanized lipoprotein metabolism, IC7Fc markedly lowered plasma triglyceride and total cholesterol levels. This was mechanistically explained by an inhibition of de novo lipogenesis in the liver, increased synthesis of bile acids from cholesterol, and down-regulated apolipoprotein B synthesis, which resulted in decreased cholesterol secretion in very low-density lipoprotein particles. As a consequence, IC7Fc treatment considerably reduced atherosclerotic lesion formation and vascular inflammation compared with current antihyperlipidemic therapy. In conclusion, IC7Fc is a promising pharmacological treatment for cardiometabolic diseases targeting hyperlipidemia and inflammation.
-
FC/FACS
Macrophage NLRP3 activation and IL-1β release drive osimertinib-induced antitumor immunity.
In Journal for Immunotherapy of Cancer on 5 October 2025 by Yu, H., Sun, X., et al.
Despite the clinical efficacy of epidermal growth factor receptor tyrosine kinase inhibitors (EGFR-TKIs) in non-small cell lung cancer (NSCLC), patient outcomes vary even among those with identical EGFR mutations. This study investigates whether osimertinib, a third-generation EGFR-TKI, activates the nucleotide-binding oligomerization domain-like receptor protein-3 (NLRP3) inflammasome in macrophages to drive antitumor immunity and explores its mechanistic basis.
Using bone marrow-derived macrophages from wild-type and gene-deficient mice, human peripheral blood mononuclear cells, and a Lewis lung cancer murine model, we assessed osimertinib-induced NLRP3 inflammasome activation, interleukin (IL)-1β secretion, pyroptosis, and tumor microenvironment (TME) remodeling. Mechanistic studies evaluated lysosomal dysfunction, calcium overload, mitochondrial damage, and reactive oxygen species (ROS) production. Clinical correlations were analyzed in patients with NSCLC treated with EGFR-TKIs.
Osimertinib triggered NLRP3 inflammasome activation in macrophages via lysosomal dysfunction-induced calcium overload, leading to mitochondrial damage and ROS production, which acted as damage-associated molecular patterns to activate NLRP3. This process promoted IL-1β release, pyroptosis, and CD8+ T-cell activation while suppressing regulatory T cells in the TME. In murine models, osimertinib's antitumor effects were abrogated by NLRP3 inhibition (MCC950) and enhanced by recombinant IL-1β (rIL-1β) co-administration (p<0.01). Clinically, high NLRP3 and IL-1β expression in tumor-associated macrophages (TAMs) correlated with prolonged progression-free survival (p<0.01) and overall survival (p<0.01) in EGFR-TKI-treated patients with NSCLC.
Osimertinib exerts off-target immunomodulatory effects by activating the tumor-extrinsic NLRP3 inflammasome, linking mitochondrial-lysosomal crosstalk to antitumor immunity. NLRP3 and IL-1β in TAMs emerge as predictive biomarkers for EGFR-TKI efficacy, while rIL-1β combination therapy represents a novel strategy to enhance clinical outcomes.
© Author(s) (or their employer(s)) 2025. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ Group.
-
Immunology and Microbiology
In Journal of Virology on 23 September 2025 by Schramm, N. J., Kovarova, M., et al.
No effective therapy or vaccine exists to protect against the next Zika virus (ZIKV) outbreak. ZIKV has been detected in multiple organs of infected people, including immune-privileged sites like the brain, eyes, and reproductive tract. ZIKV replication in the reproductive tract is of high concern; ZIKV can be transmitted sexually or to the developing fetus of pregnant women, resulting in severe congenital defects. Here, we show that ZIKV-infected immunocompetent mice rapidly controlled viremia with no viral rebound following T cell depletion. In contrast, mice genetically deficient in B and T cells (immunodeficient mice) supported sustained ZIKV replication in all tissues examined. Treatment of ZIKV-infected immunodeficient mice with the novel nucleoside analog 7-deaza-7-fluoro-2'-C-methyladenosine (DFMA) significantly reduced viremia and prolonged survival, validating immunodeficient mice for efficacy studies of ZIKV prevention and therapeutic approaches. Importantly, we demonstrate that treatment of ZIKV-infected animals with a dengue virus cross-neutralizing antibody (EDE1, C10) suppressed systemic ZIKV replication, including in the brain, eye, and male and female reproductive tracts.IMPORTANCESince 2007, Zika virus (ZIKV) infections have been documented in over 80 countries and territories, resulting in two major outbreaks thus far. ZIKV has been detected in multiple organs of infected people, including immune-privileged sites like the brain, eyes, and reproductive tract. ZIKV replication in the reproductive tract is of high concern as ZIKV can be transmitted sexually or to the developing fetus of pregnant women, resulting in severe congenital defects. Currently, no effective therapy or vaccine exists to protect against the next outbreak. Here, we developed a preclinical animal model for ZIKV infection that we used to evaluate the efficacy of a dengue virus cross-neutralizing antibody for prevention/treatment of ZIKV infection. The antibody suppressed virus replication in blood and tissues, including the reproductive tract, suggesting that passive administration of ZIKV neutralizing antibodies could be used during future ZIKV outbreaks in high-risk populations to prevent ZIKV transmission.
-
FC/FACS
-
Immunology and Microbiology
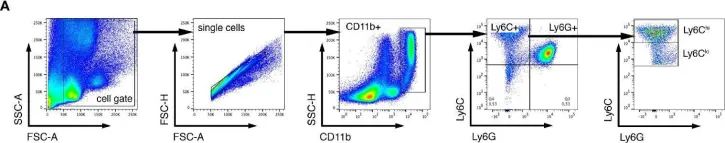
In Front Immunol on 23 July 2021 by Petermann, M., Orfanos, Z., et al.
Fig.3.A
-
FC/FACS
-
Collected and cropped from Frontiers in Immunology by CiteAb, provided under a CC-BY license
Image 1 of 7
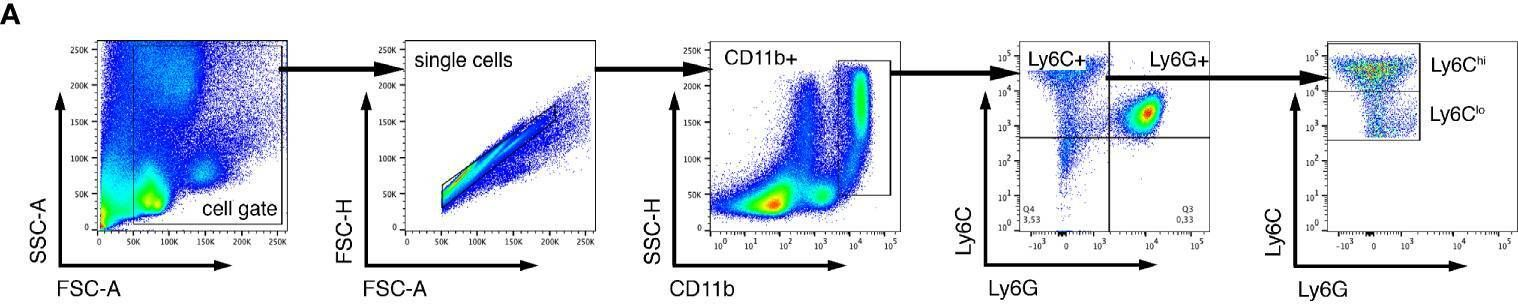
In Front Cell Neurosci on 16 March 2021 by Kim, J., Kim, J. H., et al.
Fig.3.A
-
FC/FACS
-
Mus musculus (House mouse)
Collected and cropped from Frontiers in Cellular Neuroscience by CiteAb, provided under a CC-BY license
Image 1 of 7
In PLoS One on 13 February 2021 by Gaurav, R., Mikuls, T. R., et al.
Fig.8.A
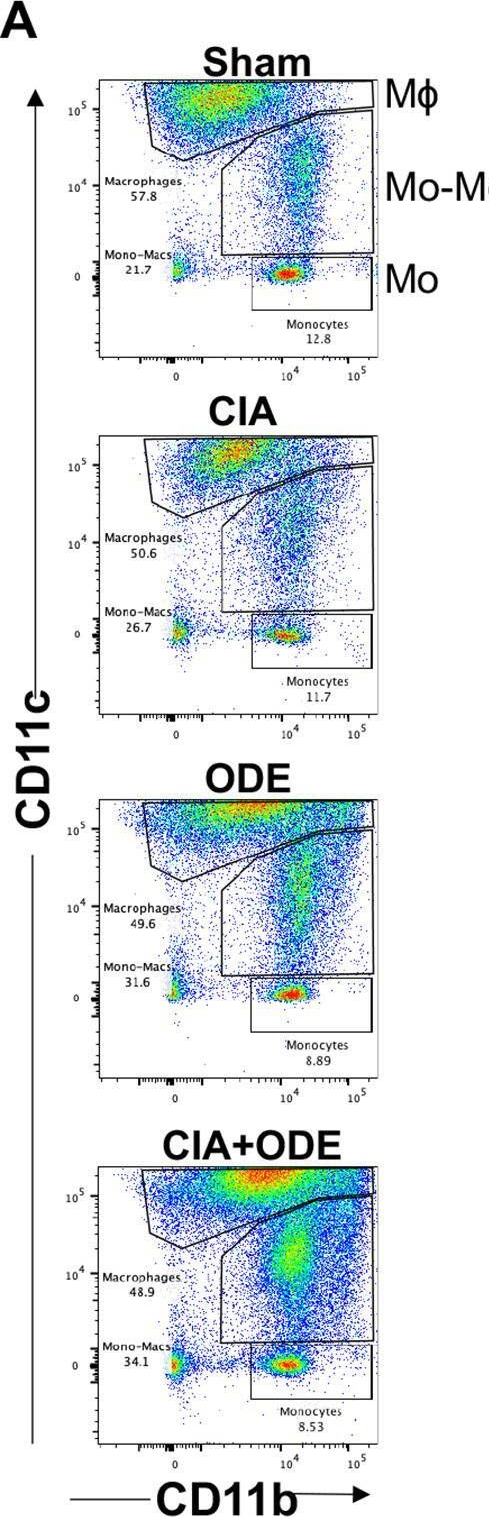
-
FC/FACS
-
Collected and cropped from PLoS ONE by CiteAb, provided under a CC-BY license
Image 1 of 7
In PLoS One on 6 September 2017 by Pierce, A. A., Duwaerts, C. C., et al.
Fig.1.A
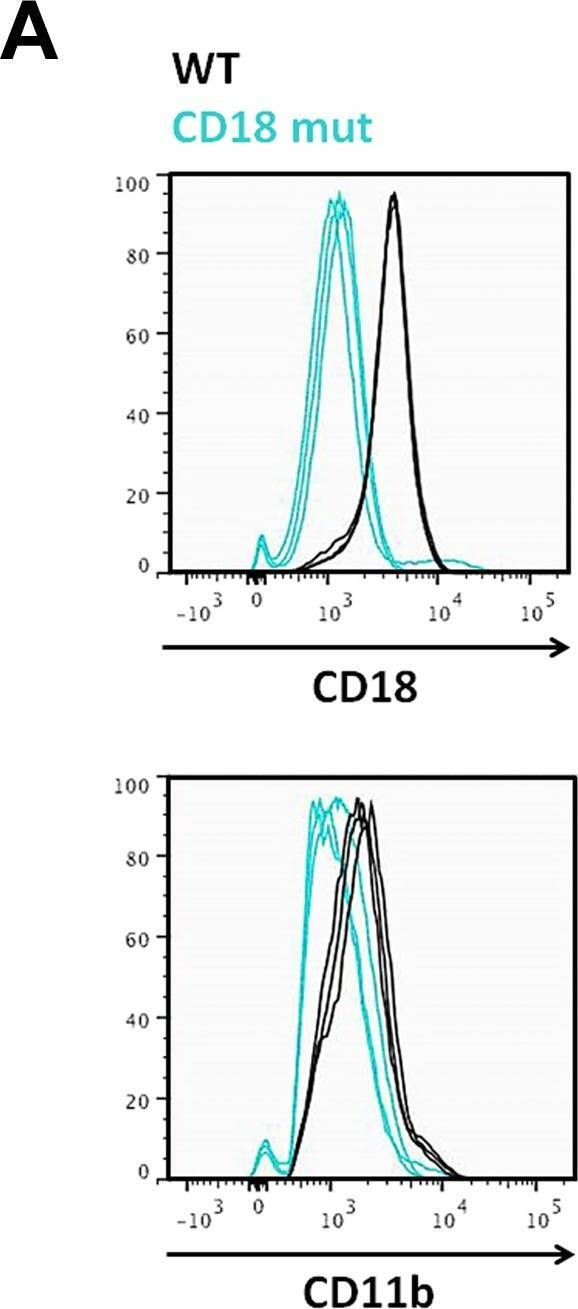
-
FC/FACS
-
Mus musculus (House mouse)
Collected and cropped from PLoS ONE by CiteAb, provided under a CC-BY license
Image 1 of 7
In PLoS One on 2 February 2016 by Kicielińska, J., Szczygieł, A., et al.
Fig.4.A
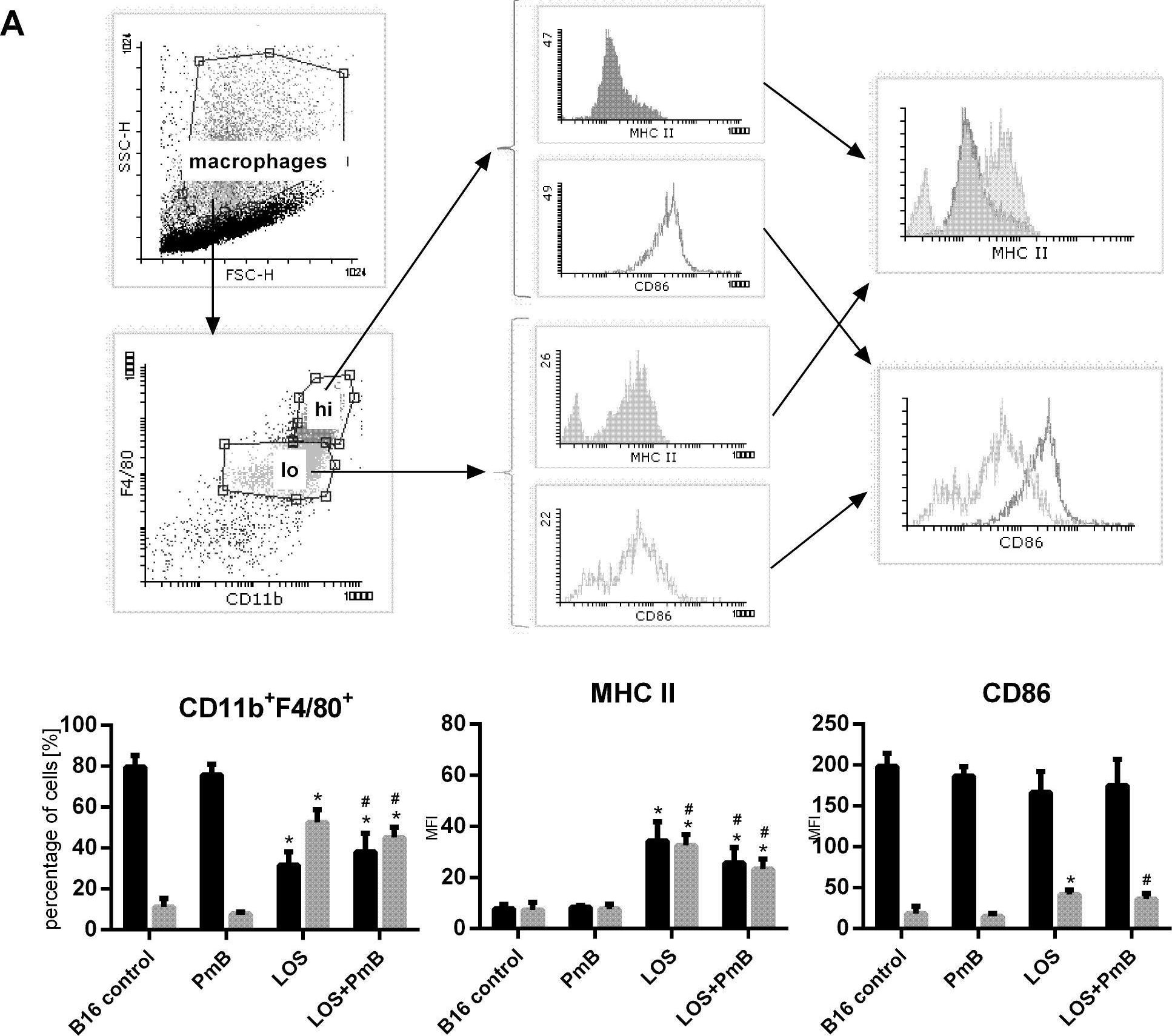
-
FC/FACS
-
Collected and cropped from PLoS ONE by CiteAb, provided under a CC-BY license
Image 1 of 7
In ISRN Endocrinol on 11 July 2013 by Darrow, A. L., Maresh, J. G., et al.
Fig.5.A
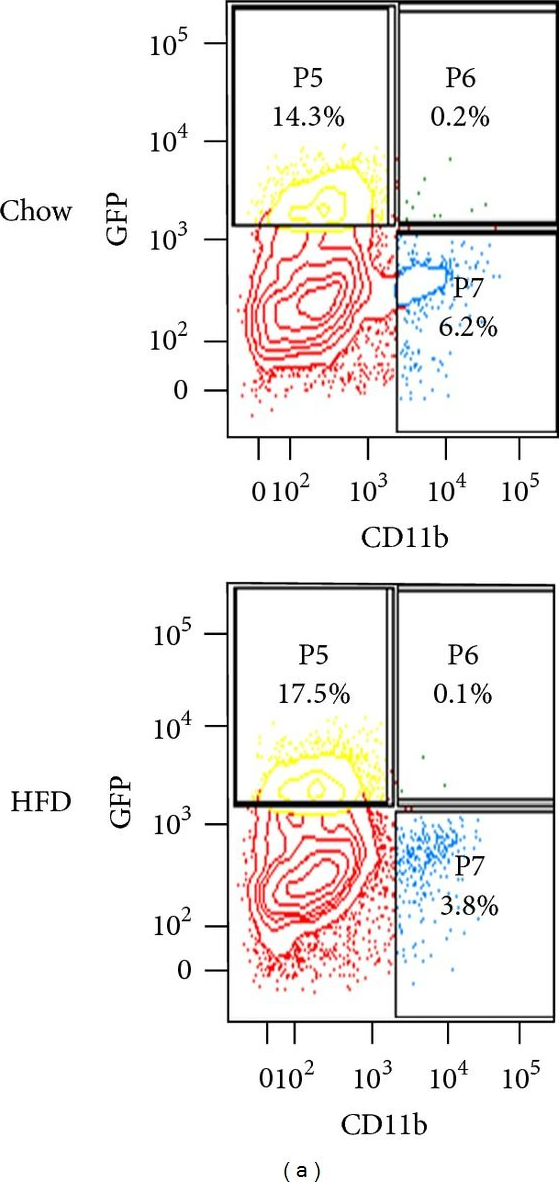
-
FC/FACS
-
Mus musculus (House mouse)
Collected and cropped from ISRN Endocrinology by CiteAb, provided under a CC-BY license
Image 1 of 7
In ISRN Endocrinol on 11 July 2013 by Darrow, A. L., Maresh, J. G., et al.
Fig.5.B
-
FC/FACS
-
Mus musculus (House mouse)
Collected and cropped from ISRN Endocrinology by CiteAb, provided under a CC-BY license
Image 1 of 7