The tumor immune microenvironment plays a critical role in tumor progression and responses to immunotherapy. Nevertheless, its cellular complexity and heterogeneity remain incompletely understood. In this study, we employed high-resolution single-cell RNA sequencing on CD45+ immune cells isolated from ten syngeneic murine tumor models, representing seven distinct cancer types under treatment-naïve conditions, thereby enabling a comprehensive profiling of tumor-infiltrating immune cells. We identified seven principal immune cell populations and provided an in-depth characterization of T cells, NK/innate lymphoid cells, dendritic cells, monocytes/macrophages, and neutrophils. Cross-species analyses further delineated conserved immune cell states and transcriptomic features within the T cell and monocyte/macrophage compartments that are shared across syngeneic models and human tumors. To investigate the functional relevance of the predominant monocyte/macrophage compartment and the notable presence of neutrophils in syngeneic tumors, we evaluated responses to anti-PD-1 therapy across various models and analyzed the enrichment of monocyte/macrophage subsets in tumors that responded to treatment. Furthermore, we conducted neutrophil depletion experiments using anti-Ly6G antibodies, administered both as monotherapy and in combination with PD-1 blockade. Remarkably, an interferon-stimulated gene-high (ISGhigh) monocyte subset was significantly enriched in models responsive to anti-PD-1 therapy. Neutrophil depletion resulted in variable antitumor effects across models but failed to enhance the efficacy of PD-1 blockade. In summary, our single-cell profiling offered a detailed atlas of the immune microenvironment across multiple syngeneic mouse tumor models, thereby enabling rational model selection for immuno-oncology studies. We uncovered an ISGhigh monocyte subset enriched in anti-PD-1 responsive models, and showed the context-dependent effects of neutrophil depletion on tumor immunity and immunotherapy, underscoring the heterogeneity and functional divergence of immune cell sublineages.
Copyright © 2025 Wang, Jiang, Deng, Yan, Zhang, Jin and Shen.
Product Citations: 171
Single-cell atlas of the tumor immune microenvironment across syngeneic murine models.
In Frontiers in Immunology on 1 December 2025 by Wang, J., Jiang, B., et al.
-
Cancer Research
-
Immunology and Microbiology
In Journal of Nanobiotechnology on 27 November 2025 by Xu, S., Yang, N., et al.
Pancreatic ductal adenocarcinoma (PDAC) remains one of the most lethal malignancies, marked by extensive stromal fibrosis and a profoundly immunosuppressive, immune-excluded tumor microenvironment (TME) that hampers the efficacy of immune checkpoint blockade. Although photodynamic therapy (PDT) can induce immunogenic cell death (ICD) and stimulate anti-tumor immunity, its effectiveness against PDAC is limited by insufficient immune activation and persistent stromal-mediated immunosuppression. To address these challenges, we develop a liposomal nanodrug that co-encapsulates a mitochondrial-targeted photosensitizer (MP) and a TGF-β receptor inhibitor (LY2109761) to synergize PDT with PD-1 checkpoint blockade. MP selectively accumulates in mitochondria and, upon light activation, amplifies mitochondrial reactive oxygen species production, inducing mitochondrial damage. This damage triggers the release of mitochondrial DNA and damage-associated molecular patterns, activating the STING pathway and eliciting potent ICD and anti-tumor immune responses. Simultaneously, liposome-mediated delivery of LY2109761 mitigates stromal desmoplasia and reverses TGF-β-driven immune suppression, enhancing effector T cell infiltration and activity. In murine PDAC models, this dual-action strategy transforms the immune-cold TME into an immune-inflamed phenotype, sensitizing tumors to PD-1 therapy and leading to pronounced tumor regression and prolonged survival. Our findings present a promising nanodrug-based approach to remodel the fibrotic and immunosuppressive TME of PDAC and enhance immunotherapeutic outcomes.© 2025. The Author(s).
-
Cancer Research
-
Cell Biology
In Advanced Science (Weinheim, Baden-Wurttemberg, Germany) on 1 November 2025 by Xu, B., Wu, Y. M., et al.
Integrating mild hyperthermia (MH) with 125I brachytherapy holds potential for overcoming treatment resistance and improving anticancer efficacy. Here, magnetic nanoparticles (MNPs) with a suitable Curie temperature are constructed and incorporated with silver rods coated with 125I to form composite seeds. In vitro simulations and in vivo validations demonstrated their effective performance in radiation dose and temperature control. Compared with traditional thermoseeds and previously reported MNPs, this composite seed exhibits direction-independent and self-regulated heating efficiency. Additionally, the titanium shell prevented MNPs leakage and enabled its repeated hyperthermia treatment capacity. Subsequently, the enhanced pancancer anticancer efficacy of the composite seed-relied 125I@MH therapy is confirmed through cellular and animal experiments involving liver cancer and prostate cancer. Further tumor microenvironment investigations based on a subcutaneous liver cancer mouse model identified that 125I therapy recruited Cd274/Pd-l1+ neutrophils and induced T-cell exhaustion, leading to immune evasion and brachytherapy resistance. The addition of MH significantly reversed this effect, restoring the function of effector T cells (IFN-γ+ T cells) and activating T-cell immunity. In conclusion, this study developed a novel composite seed with superior anticancer efficacy, which holds promising therapeutic potential for the treatment of malignancies, particularly solid tumors, in future clinical practice.
© 2025 The Author(s). Advanced Science published by Wiley‐VCH GmbH.
-
Immunology and Microbiology
-
Plant Science
In Journal for Immunotherapy of Cancer on 23 October 2025 by Zhang, Y., Yu, R., et al.
Despite progress in immunotherapy for several solid tumors, pancreatic ductal adenocarcinoma (PDAC) remains largely unresponsive, primarily due to its profoundly immunosuppressive tumor microenvironment (TME) characterized by limited CD8+ T cell infiltration. Novel strategies are needed to overcome this immune resistance and enhance the efficacy of checkpoint blockade.
We established a patient-derived organoid (PDO)-autologous T cell co-culture platform using endoscopic ultrasound-guided fine-needle aspiration (EUS-FNA) specimens from patients with PDAC unresponsive to anti-programmed cell death protein-1 (PD-1) therapy. This high-throughput system was used to screen a focused library of epigenetic compounds. The effects of candidate drugs were validated in orthotopic PDAC models, integrating functional assays, sequencing analyses, and patient data.
Through screening, we identified the histone demethylase inhibitor JIB04, which synergized with anti-PD-1 therapy to enhance T cell cytotoxicity in PDO-T cell co-cultures. Mechanistically, JIB04 suppressed nuclear-factor-E2-related factor 2 (Nrf2) and reduced chromatin accessibility at distal regulatory regions of its downstream target solute carrier family 40 member 1 (Slc40a1), impairing iron efflux and promoting ferroptosis in tumor cells. This ferroptotic stress facilitated CD8+ T cell infiltration and activation, thereby converting the PDAC TME from "cold" to "hot." Patients with PDAC with lower Nrf2 and Slc40a1 expression exhibited higher CD8+ T cell infiltration and improved responses to anti-PD-1 therapy.
Our findings establish a PDO-T cell platform for precision immunotherapy screening and identify JIB04 as a promising epigenetic agent that induces ferroptosis and sensitizes PDAC to immune checkpoint blockade. This ferroptosis-based reprogramming provides a potential strategy to overcome resistance and improve clinical outcomes in PDAC.
© Author(s) (or their employer(s)) 2025. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ Group.
-
FC/FACS
-
Cancer Research
-
Genetics
-
Immunology and Microbiology
Iron overload in CD11c+ myeloid cells exacerbates acetaminophen hepatotoxicity.
In Communications Biology on 21 October 2025 by Liu, S., Kanamori, Y., et al.
Acute liver injury often progresses to liver failure, with immune responses playing a critical role in regulating the inflammatory process. In this study, we aim to investigate the effects of iron on CD11c+ myeloid cells during acetaminophen-induced liver injury. Iron overload caused by F-box and leucine-rich repeat protein 5 (FBXL5) deficiency in CD11c+ cells exacerbates liver damage. CD11c+ myeloid cell-specific FBXL5-deficient mice exhibit higher serum transaminase levels, liver injury, and mortality than the controls. These phenotypes are associated with enhanced neutrophil infiltration and expression of interleukin (IL)-6, a pro-inflammatory cytokine. Mechanistically, iron overload in FBXL5-deficient cells increases IL-6 production by facilitating the recruitment of nuclear factor-κB to the Il-6 promoter. In vivo, IL-6 neutralization mitigates liver injury, confirming its role in disease progression. Our findings highlight the role of iron in immune responses and suggest that targeting iron may represent a potential therapeutic strategy for liver injury.
© 2025. The Author(s).
-
FC/FACS
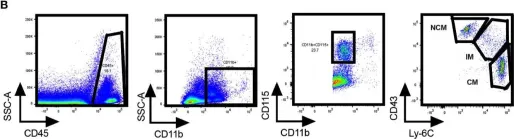
In Front Immunol on 17 November 2020 by Consiglio, C. R. & Gollnick, S. O.
Fig.2.B
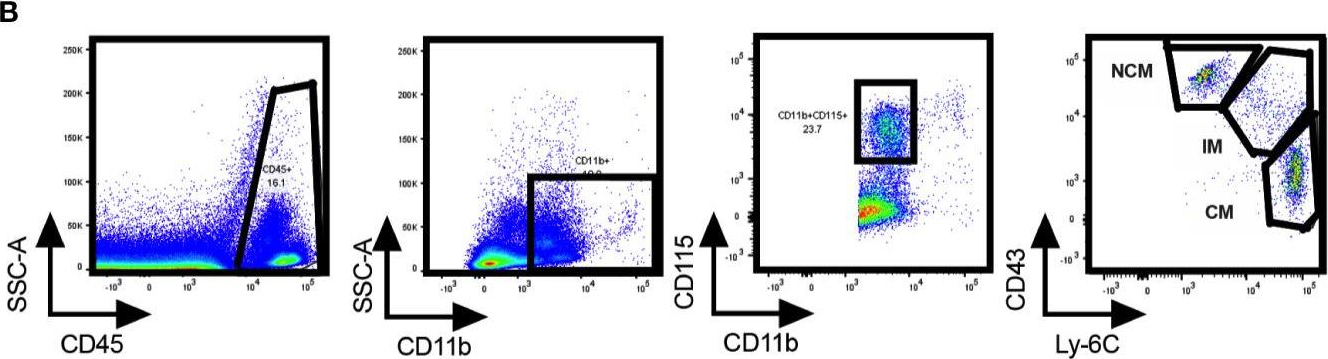
-
FC/FACS
-
Mus musculus (House mouse)
Collected and cropped from Frontiers in Immunology by CiteAb, provided under a CC-BY license
Image 1 of 2
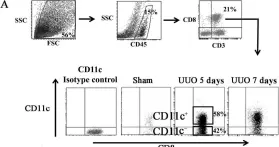
In Int J Mol Sci on 22 December 2016 by Wang, H., Wang, J., et al.
Fig.5.A
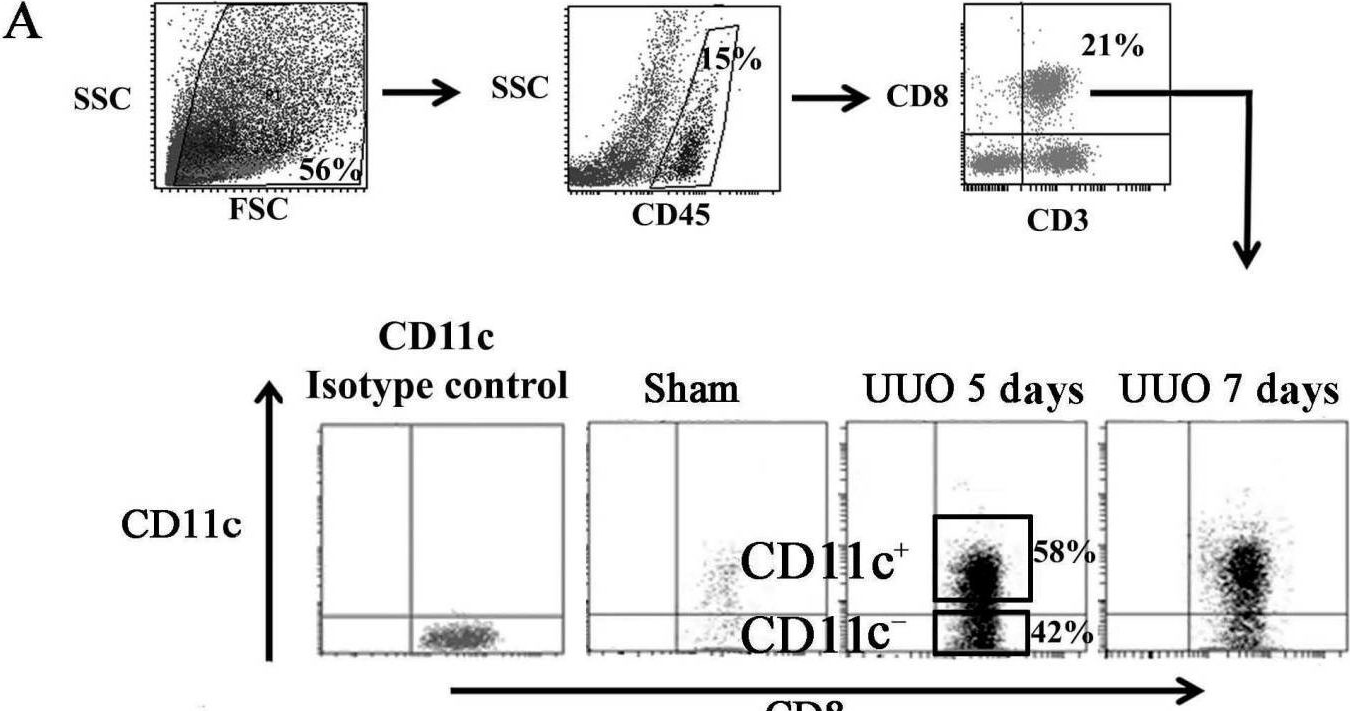
-
FC/FACS
-
Collected and cropped from International Journal of Molecular Sciences by CiteAb, provided under a CC-BY license
Image 1 of 2