Assessing the long-term efficacy of MPXV vaccine candidates is crucial for the global response to the ongoing mpox epidemic. Built upon our previous study of the mpox quadrivalent mRNA vaccine, herein we reported that MPXV-1103 could elicit sustained humoral and cellular immunity in mice, including the induction of MPXV A35/B6/A29/M1-specific IgG antibodies, VACV neutralizing antibodies and activated cytotoxic CD8+T cells, which provides 100% protection against lethal VACV challenge even at 280 days after the first vaccination. Our results provide critical insights for orthopoxvirus vaccine development.
Product Citations: 208
In Emerging Microbes Infections on 1 December 2025 by Li, E., Yang, Q., et al.
-
Genetics
-
Immunology and Microbiology
Cancer cells accelerate exhaustion of persistently activated mouse CD4+ T cells.
In Oncoimmunology on 1 December 2025 by Stachowiak, M., Becker, W., et al.
Most exhaustion studies have focused on CD8+ T cells. Here, we demonstrated reciprocal growth inhibition of CD4+ T cells and colorectal cancer cells, which induced the expression of PD-1, PD-L1, and PD-L2 in CD4+ T cells. The accelerated exhaustion of CD4+ T cells was evidenced by the reduced secretion of several cytokines, including IL-2, IFN-γ, or TNFα, and elevated secretion of CXCL family chemokines. Progressive expression of PD-L1, CTLA4, and IDO1 exhaustion markers occurred concomitantly with tumor growth in vivo in a mouse model. The pattern of CD4+ T cell exhaustion was analogous to that observed in CD8+ T cells, although with altered dynamics. The PD-L1-high phenotype can be induced by co-culture with tumor cells and is mediated by secreted factors in addition to cell contact. Our findings revealed that IFN-γ receptor knockout T cells exhibited PD-L1 protein expression when cultured with tumor cells, suggesting that PD-L1 expression is not fully dependent on IFN-γ. The TIL population undergoing exhaustion due to persistent antigen stimulation in the presence of cancer cells gradually acquires an immunosuppressive phenotype. The accumulation of inhibitory signals exerted by both cancer cells and T cells, which had converted to a suppressive phenotype, accelerated T cell exhaustion.
-
Cancer Research
-
Immunology and Microbiology
In Frontiers in Immunology on 17 July 2025 by Guldenpfennig, C., Guan, Y., et al.
Upon antigen recognition, naive CD8 T cells must induce c-JUN N-terminal kinase (JNK), NF-κB, and Akt signaling to drive differentiation and generate a heterogeneous effector response. While the roles of these three pathways individually in mediating essential cellular responses for CD8 T cell differentiation are well established, the mechanisms of signal integration and crosstalk between these pathways to produce a diverse and heterogeneous response to infection remain poorly understood. Here, we establish the critical role of the Plenty of SH3 Domains (POSH) scaffold protein in coordinating signals from all three pathways to support CD8 T cell differentiation and fate.
Using novel conditional T cell POSH knockout reporter mouse models (as POSHfl/fl CD4-Cre eGFP, POSHfl/fl GzmB-Cre eGFP), we determined the phenotype of T cells in the thymus and periphery through flow cytometry. Polyclonal and OT1 TCR transgenic POSH cKO CD8 T cells were stimulated in vitro and analyzed by flow cytometry to assess cell fate. JNK, NF-κB, and Akt pathways were examined via flow cytometry and immunoblotting. Purified OT1 CD8 T cells from these mice were adoptively transferred and subsequently challenged with VSV-OVA infection; their phenotype, effector function, and signaling were then assessed ex vivo by flow cytometry.
We demonstrate that POSH is essential for proper induction of the JNK, NF-κB, and Akt pathways. Furthermore, the absence of these signals due to POSH deficiency results in reduced differentiation into short-lived effector cells (SLECs), delayed proliferation, and decreased survival of memory precursor cells (MPECs) during the contraction phase.
Collectively, these data identify POSH as a key regulator of CD8 T cell fate and enhance our understanding of the complex mechanisms governing signal integration during CD8 T cell responses to infection.
Copyright © 2025 Guldenpfennig, Guan, Cseri, Lopez, Teixeiro and Daniels.
-
Immunology and Microbiology
In Nature Communications on 1 July 2025 by Perrotta, M., Perrotta, S., et al.
Activated immune cells infiltrate the vasculature during the pathophysiology of hypertension by establishing a vascular-immune interface that contributes to blood pressure dysregulation and organ failure. Many observations indicate a key role of CD8+ T cells in hypertension but mechanisms regulating their activation and interplay with the cardiovascular system are still unknown. In murine model, here we show that a specific member of the phosphoinositide-3-kinases (PI3K) family of lipid kinases, PI3Kγ, is a key intracellular signaling of CD8+ T cells activation and RANTES/CCL5 secretion in hypertension: CCL5-CCR5 signaling is crucial for the establishment of the vascular-immune interface in peripheral organs, lastly contributing to CD8+ tissue infiltration, organ dysfunction and blood pressure elevation. Our studies identify PI3Kγ as a booster of effector CD8+ T cell function, even in the absence of external stimuli. Lastly, an enhanced PI3Kγ signaling mediates the bystander activation of CD8+ T cells and proves effective in transferring the hypertensive phenotype between mice.
© 2025. The Author(s).
-
Cardiovascular biology
-
Immunology and Microbiology
Dual asparagine-depriving nanoparticles against solid tumors.
In Nature Communications on 1 July 2025 by Shen, Y., Wang, H., et al.
Depletion of circulatory asparagine (Asn) by L-asparaginase (ASNase) has been used for clinical treatment of leukemia, whereas solid tumors are unresponsive to this therapy owing to their active Asn biosynthesis. Herein, we develop a type of core-shell structured cascade-responsive nanoparticles (NPs) for sequential modulation of exogenous Asn supply and endogenous Asn production. The reactive oxygen species-sensitive NP shells disintegrate in the tumor microenvironment and liberate ASNase to scavenge extracellular Asn. The acid-labile NP cores subsequently decompose in the tumor cells and release rotenone to block intracellular Asn biosynthesis. Administration of the dual Asn-depriving NPs in murine models of triple-negative breast cancer and colorectal cancer substantially suppress the growth and epithelial-mesenchymal transition of primary and relapsed tumors, fully eradicate spontaneous and post-surgical metastasis, and confer long-term T cell memory for complete resistance to tumor rechallenge. This study represents a generalized strategy to harness amino acid depletion therapy against solid tumors.
© 2025. The Author(s).
-
Cancer Research
In Front Immunol on 25 April 2020 by Rana, A., de Almeida, F. C., et al.
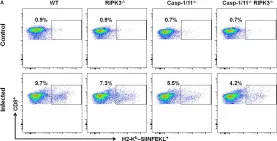
Fig.2.A

-
FC/FACS
-
Mus musculus (House mouse)
Collected and cropped from Front Immunol by CiteAb, provided under a CC-BY license
Image 1 of 6
In Front Immunol on 25 April 2020 by Rana, A., de Almeida, F. C., et al.
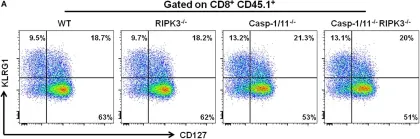
Fig.6.A

-
FC/FACS
-
Mus musculus (House mouse)
Collected and cropped from Front Immunol by CiteAb, provided under a CC-BY license
Image 1 of 6
In Front Immunol on 25 April 2020 by Rana, A., de Almeida, F. C., et al.
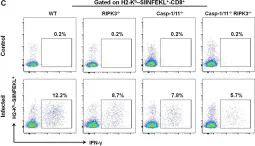
Fig.5.C

-
FC/FACS
-
Mus musculus (House mouse)
Collected and cropped from Front Immunol by CiteAb, provided under a CC-BY license
Image 1 of 6
In Front Immunol on 25 April 2020 by Rana, A., de Almeida, F. C., et al.
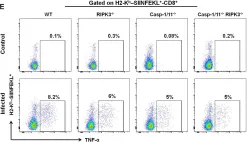
Fig.5.E

-
FC/FACS
-
Mus musculus (House mouse)
Collected and cropped from Front Immunol by CiteAb, provided under a CC-BY license
Image 1 of 6
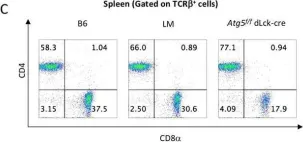
In Sci Rep on 13 April 2018 by Murera, D., Arbogast, F., et al.
Fig.1.C

-
FC/FACS
-
Mus musculus (House mouse)
Collected and cropped from Sci Rep by CiteAb, provided under a CC-BY license
Image 1 of 6
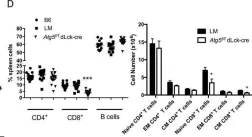
In Sci Rep on 13 April 2018 by Murera, D., Arbogast, F., et al.
Fig.1.D

-
FC/FACS
-
Mus musculus (House mouse)
Collected and cropped from Sci Rep by CiteAb, provided under a CC-BY license
Image 1 of 6