Botanical extracts are recognized in traditional medicine for their therapeutic potential and safety standards. Botanical extracts are viable and sustainable alternatives to synthetic drugs, being essential in drug discovery for various diseases. Senna septemtrionalis (Viv.) H.S. Irwin & Barneby is a medical plant traditionally used to treat inflammation. However, its antioxidant and anti-inflammatory properties and the molecular pathways activated in microglial cells require further investigation. Therefore, this study examines the antioxidant and anti-inflammatory properties of Senna septemtrionalis (Viv.) H.S. Irwin & Barneby methanol extracts (SMEs) in lipopolysaccharide (LPS)-stimulated mouse microglial cells. SMEs significantly inhibit LPS-induced nitric oxide (NO) and proinflammatory cytokine production, which are mediated through the dephosphorylation of mitogen-activated protein kinases and inhibition of nuclear factor kappa B (NF-κB) translocation into the nucleus. Additionally, SME treatment upregulated the expression of nuclear factor erythroid 2-related factor 2 (Nrf2) and heme oxygenase (HO)-1, reducing oxidative stress, indicated by a decrease in reactive oxygen species and restoration of the total glutathione content in LPS-stimulated BV2 cells. The inhibitory effects of SMEs on inflammatory mediator production and NF-κB nuclear translocation were significantly reversed by Sn-protoporphyrin, a specific HO-1 inhibitor. These findings demonstrate that SME protects microglial cells by activating the Nrf2/HO-1 pathway and inhibiting NF-κB translocation.
Product Citations: 93
In International Journal of Molecular Sciences on 24 February 2025 by Lim, J. S., Li, X., et al.
-
Immunology and Microbiology
-
Neuroscience
In Nature Communications on 8 February 2023 by He, Y., Ge, C., et al.
Although elevated levels of anti-citrullinated protein antibodies (ACPAs) are a hallmark of rheumatoid arthritis (RA), the in vivo functions of these antibodies remain unclear. Here, we have expressed monoclonal ACPAs derived from patients with RA, and analyzed their functions in mice, as well as their specificities. None of the ACPAs showed arthritogenicity nor induced pain-associated behavior in mice. However, one of the antibodies, clone E4, protected mice from antibody-induced arthritis. E4 showed a binding pattern restricted to skin, macrophages and dendritic cells in lymphoid tissue, and cartilage derived from mouse and human arthritic joints. Proteomic analysis confirmed that E4 strongly binds to macrophages and certain RA synovial fluid proteins such as α-enolase. The protective effect of E4 was epitope-specific and dependent on the interaction between E4-citrullinated α-enolase immune complexes with FCGR2B on macrophages, resulting in increased IL-10 secretion and reduced osteoclastogenesis. These findings suggest that a subset of ACPAs have therapeutic potential in RA.
© 2023. The Author(s).
-
Mus musculus (House mouse)
In Plants (Basel, Switzerland) on 14 November 2022 by Lim, J. S., Bae, J., et al.
Symplocos sumuntia Buch.-Ham. ex D. Don (S. sumuntia) is a traditional medicinal herb used in Asia to treat various pathologies, including cough, stomachache, tonsillitis, hypertension, and hyperlipidemia. Although the anti-inflammatory activity of S. sumuntia has been reported, little is known about its anti-inflammatory activity and molecular mechanisms in microglial cells. Therefore, we investigated the inhibitory effects of S. sumuntia methanol extract (SSME) on the inflammatory responses in lipopolysaccharide (LPS)-treated BV2 cells. The SSME significantly inhibited the LPS-stimulated inducible nitric oxide synthase and cyclooxygenase-2 expression, as well as the production of nitric oxide (NO), a proinflammatory mediator. The production of proinflammatory cytokines, including interleukin (IL)-6, tumor necrosis factor-α, and IL-1β, was suppressed by the SSME in the LPS-induced BV2 cells. The mechanism underlying the anti-inflammatory effects of SSME involves the suppression of the LPS-stimulated phosphorylation of mitogen-activated protein kinases (MAPKs) such as JNK. Moreover, we showed that the LPS-stimulated nuclear translocation of the nuclear factor-κB (NF-κB)/p65 protein, followed by IκB degradation, was decreased by the SSME treatment. Collectively, these results showed that the SSME induced anti-inflammatory effects via the suppression of the MAPK signaling pathways, accompanied by changes in the NF-κB translocation into the nucleus. Therefore, SSME may be employed as a potential therapeutic candidate for various inflammatory diseases.
-
Mus musculus (House mouse)
-
Immunology and Microbiology
-
Neuroscience
In Nature Communications on 19 April 2022 by Lee, H. J., Song, K. H., et al.
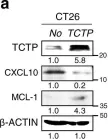
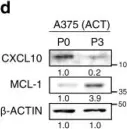
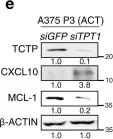
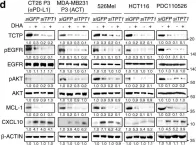
Immunotherapy has emerged as a powerful approach to cancer treatment. However, immunotherapeutic resistance limits its clinical application. Therefore, identifying immune-resistant factors, which can be targeted by clinically available drugs and it also can be a companion diagnostic marker, is needed to develop combination strategies. Here, using the transcriptome data of patients, and immune-refractory tumor models, we identify TCTP as an immune-resistance factor that correlates with clinical outcome of anti-PD-L1 therapy and confers immune-refractory phenotypes, decreased T cell trafficking to the tumor and resistance to cytotoxic T lymphocyte-mediated tumor cell killing. Mechanistically, TCTP activates the EGFR-AKT-MCL-1/CXCL10 pathway by phosphorylation-dependent interaction with Na, K ATPase. Furthermore, treatment with dihydroartenimsinin, the most effective agent impending the TCTP-mediated-refractoriness, synergizes with T cell-mediated therapy to control immune-refractory tumors. Thus, our findings suggest a role of TCTP in promoting immune-refractoriness, thereby encouraging a rationale for combination therapies to enhance the efficacy of T cell-mediated therapy.
© 2022. The Author(s).
-
WB
-
Mus musculus (House mouse)
-
Cancer Research
-
Immunology and Microbiology
In The Journal of Clinical Investigation on 15 March 2022 by Oh, S. J., Lee, H. J., et al.
Immune checkpoint blockade (ICB) therapy has shifted the paradigm for cancer treatment. However, the majority of patients lack effective responses because of the emergence of immune-refractory tumors that disrupt the amplification of antitumor immunity. Therefore, the identification of clinically available targets that restrict antitumor immunity is required to develop potential combination therapies. Here, using transcriptomic data on patients with cancer treated with programmed cell death protein 1 (PD-1) therapy and newly established mouse preclinical anti-PD-1 therapy-refractory models, we identified NANOG as a factor restricting the amplification of the antitumor immunity cycle, thereby contributing to the immune-refractory feature of the tumor microenvironment (TME). Mechanistically, NANOG induced insufficient T cell infiltration and resistance to CTL-mediated killing via the histone deacetylase 1-dependent (HDAC1-dependent) regulation of CXCL10 and MCL1, respectively. Importantly, HDAC1 inhibition using an actionable agent sensitized NANOGhi immune-refractory tumors to PD-1 blockade by reinvigorating the antitumor immunity cycle. Thus, our findings implicate the NANOG/HDAC1 axis as a central molecular target for controlling immune-refractory tumors and provide a rationale for combining HDAC inhibitors to reverse the refractoriness of tumors to ICB therapy.
-
WB
-
Cancer Research
-
Immunology and Microbiology
In Nat Commun on 19 April 2022 by Lee, H. J., Song, K. H., et al.
Fig.3.A

-
WB
-
Collected and cropped from Nat Commun by CiteAb, provided under a CC-BY license
Image 1 of 4
In Nat Commun on 19 April 2022 by Lee, H. J., Song, K. H., et al.
Fig.4.D

-
WB
-
Collected and cropped from Nat Commun by CiteAb, provided under a CC-BY license
Image 1 of 4
In Nat Commun on 19 April 2022 by Lee, H. J., Song, K. H., et al.
Fig.4.E

-
WB
-
Collected and cropped from Nat Commun by CiteAb, provided under a CC-BY license
Image 1 of 4
In Nat Commun on 19 April 2022 by Lee, H. J., Song, K. H., et al.
Fig.6.D

-
WB
-
Collected and cropped from Nat Commun by CiteAb, provided under a CC-BY license
Image 1 of 4