Clinical applications of CAR-T cells are limited by the scarcity of tumor-specific targets and are often afflicted with the same on-target/off-tumor toxicities that plague other cancer treatments. A new promising strategy to enforce tumor selectivity is the use of logic-gated, two-receptor systems. One well-described application is termed Tmod™, which originally utilized a blocking inhibitory receptor directed towards HLA-I target antigens to create a protective NOT gate. Here we show that the function of Tmod blockers targeting non-HLA-I antigens is dependent on the height of the blocker antigen and is generally compatible with small, membrane-proximal targets. We compensate for this apparent limitation by incorporating modular hinge units to artificially extend or retract the ligand-binding domains relative to the effector cell surface, thereby modulating Tmod activator and blocker function. By accounting for structural differences between activator and blocker targets, we developed a set of simple geometric parameters for Tmod receptor design that enables targeting of blocker antigens beyond HLA-I, thereby broadening the applications of logic-gated cell therapies.
Copyright © 2024 Partin, Bruno, Shafaattalab, Vander Mause, Winters, Daris, Gahrs, Jette, DiAndreth, Sandberg, Hamburger, Kamb and Riley.
Product Citations: 39
Geometric parameters that affect the behavior of logic-gated CAR T cells.
In Frontiers in Immunology on 12 February 2024 by Partin, A. C., Bruno, R., et al.
-
FC/FACS
-
Homo sapiens (Human)
-
Immunology and Microbiology
In Molecular Cancer on 6 January 2024 by Gammelgaard, O. L., Terp, M. G., et al.
Adoptive cell transfer cancer immunotherapy holds promise for treating disseminated disease, yet generating sufficient numbers of lymphocytes with anti-cancer activity against diverse specificities remains a major challenge. We recently developed a novel procedure (ALECSAT) for selecting, expanding and maturating polyclonal lymphocytes from peripheral blood with the capacity to target malignant cells.
Immunodeficient mice were challenged with triple-negative breast cancer cell lines or patient-derived xenografts (PDX) and treated with allogeneic or autologous ALECSAT cells with and without anti-PDL1 therapy to assess the capacity of ALECSAT cells to inhibit primary tumor growth and metastasis.
ALECSAT mono therapy inhibited metastasis, but did not inhibit primary tumor growth or prolong survival of tumor-bearing mice. In contrast, combined ALECSAT and anti-PDL1 therapy significantly inhibited primary tumor growth, nearly completely blocked metastasis, and prolonged survival of tumor-bearing mice.
Combined ALECSAT and anti-PDL1 therapy results in favorable anti-cancer responses in both cell line-derived xenograft and autologous PDX models of advanced triple-negative breast cancer.
© 2023. The Author(s).
-
FC/FACS
-
Cancer Research
Robust In Vitro Pharmacology of Tmod, a Synthetic Dual-Signal Integrator for Cancer Cell Therapy.
In Frontiers in Immunology on 2 April 2022 by Manry, D., Bolanos, K., et al.
Progress toward improved solid-tumor treatment has long been hindered by the lack of truly tumor-specific targets. We have developed an approach to T cell therapy based on a dual-receptor system called Tmod™ that addresses this problem. The Tmod system exploits one of the few common genetic differences between tumor and normal cells: loss of heterozygosity (LOH). It utilizes the basic mechanistic logic that evolved in early vertebrates to mediate self vs. non-self discrimination, where an activation stimulus is blocked by self-ligands. Tmod constructs employ a chimeric antigen receptor (CAR) or T cell receptor (TCR) as activator component and a modified LIR-1 inhibitory receptor (blocker) to achieve high selectivity based on expression of the blocker antigen (Ag). Here we explore the in vitro pharmacology of a blocker directed at the HLA-A*02 Ag paired with either a mesothelin CAR or an HLA-A*11-restricted KRAS peptide TCR. While more sensitive to receptor expression changes on effector cells, we show that Tmod response is well-buffered against variations in Ag levels on target cells. In addition, the data reveal at least two distinguishable pharmacologic mechanisms of Tmod blocker function: (1) reducing activator sensitivity and (2) decreasing activation magnitude.
Copyright © 2022 Manry, Bolanos, DiAndreth, Mock and Kamb.
-
FC/FACS
-
Homo sapiens (Human)
-
Cancer Research
-
Immunology and Microbiology
-
Pharmacology
In Cancer Immunology, Immunotherapy : CII on 1 July 2020 by Teck, A. T., Urban, S., et al.
Cyclin A1 is a promising antigen for T cell therapy being selectively expressed in high-grade ovarian cancer (OC) and acute myeloid leukemia (AML) stem cells. For adoptive T cell therapy, a single epitope has to be selected, with high affinity to MHC class I and adequate processing and presentation by malignant cells to trigger full activation of specific T cells. In silico prediction with three algorithms indicated 13 peptides of Cyclin A1 9 to 11 amino acids of length to have high affinity to HLA-A*02:01. Ten of them proved to be affine in an HLA stabilization assay using TAP-deficient T2 cells. Their immunogenicity was assessed by repetitive stimulation of CD8+ T cells from two healthy donors with single-peptide-pulsed dendritic cells or monocytes. Intracellular cytokine staining quantified the enrichment of peptide-specific functional T cells. Seven peptides were immunogenic, three of them against both donors. Specific cell lines were cloned and used in killing assays to demonstrate recognition of endogenous Cyclin A1 in the HLA-A*02:01-positive AML cell line THP-1. Immunopeptidome analysis based on direct isolation of HLA-presented peptides by mass spectrometry of primary AML and OC samples identified four naturally presented epitopes of Cyclin A1. The immunopeptidome of HeLa cells transfected with Cyclin A1 and HLA-A*02:01 revealed six Cyclin A1-derived HLA ligands. Epitope p410-420 showed high affinity to HLA-A*02:01 and immunogenicity in both donors. It proved to be naturally presented on primary AML blast and provoked spontaneous functional response of T cells from treatment naïve OC and, therefore, warrants further development for clinical application.
-
Cancer Research
-
Immunology and Microbiology
In Journal of Inflammation (London, England) on 27 February 2020 by Shao, G., Liu, Q., et al.
Prediction and identification of cytotoxic T lymphocyte (CTL) epitopes from tumor associated antigens is a crucial step for the development of tumor immunotherapy strategy. Endocan has been identified as antigen overexpressed in various tumors.
In this experiment, we predicted and identified HLA-A2-restricted CTL epitopes from endocan by using the following procedures. Firstly, we predicted the epitopes from the amino acid sequence of endocan by computer-based methods; Secondly, we determined the affinity of the predicted peptide with HLA-A2.1 molecule by peptide-binding assay; Thirdly, we elicited the primary T cell response against the predicted peptides in vitro; Lastly, we tested the specific CTLs toward endocan and HLA-A2.1 positive target cells.
These data demonstrated that peptides of endocan containing residues 4-12 and 9-17 could elicit specific CTLs producing interferon-γ and cytotoxicity.
Therefore, our findings suggested that the predicted peptides were novel HLA-A2.1-restricted CTL epitopes, and might provide promising target for tumor immunotherapy.
© The Author(s) 2020.
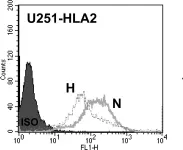
In PLoS One on 8 September 2012 by Ge, L., Cornforth, A. N., et al.
Fig.7.A

-
FC/FACS
-
Homo sapiens (Human)
Collected and cropped from PLoS One by CiteAb, provided under a CC-BY license
Image 1 of 4
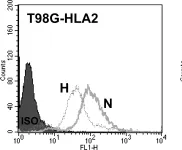
In PLoS One on 8 September 2012 by Ge, L., Cornforth, A. N., et al.
Fig.7.B

-
FC/FACS
-
Homo sapiens (Human)
Collected and cropped from PLoS One by CiteAb, provided under a CC-BY license
Image 1 of 4
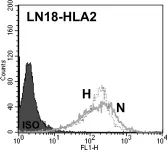
In PLoS One on 8 September 2012 by Ge, L., Cornforth, A. N., et al.
Fig.7.C

-
FC/FACS
-
Homo sapiens (Human)
Collected and cropped from PLoS One by CiteAb, provided under a CC-BY license
Image 1 of 4
In PLoS One on 8 September 2012 by Ge, L., Cornforth, A. N., et al.
Fig.7.D

-
FC/FACS
-
Homo sapiens (Human)
Collected and cropped from PLoS One by CiteAb, provided under a CC-BY license
Image 1 of 4