The objective of this study was to investigate the efficacy of CRISPR/Cas9-mediated A4GALT suppression in rescuing endothelial dysfunction in Fabry disease (FD) endothelial cells (FD-ECs) derived from human induced pluripotent stem cells (hiPSCs).
We differentiated hiPSCs (WT (wild-type), WTC-11), GLA-mutant hiPSCs (GLA-KO, CMC-Fb-002), and CRISPR/Cas9-mediated A4GALT-KO hiPSCs (GLA/A4GALT-KO, Fb-002-A4GALT-KO) into ECs and compared FD phenotypes and endothelial dysfunction. We also analyzed the effect of A4GALT suppression on reactive oxygen species (ROS) formation and transcriptome profiles through RNA sequencing.
GLA-mutant hiPSC-ECs (GLA-KO and CMC-Fb-002) showed downregulated expression of EC markers and significantly reduced α-GalA expression with increased Gb-3 deposition and intra-lysosomal inclusion bodies. However, CRISPR/Cas9-mediated A4GALT suppression in GLA/A4GALT-KO and Fb-002-A4GALT-KO hiPSC-ECs increased expression levels of EC markers and rescued these FD phenotypes. GLA-mutant hiPSC-ECs failed to form tube-like structure in tube formation assays, showing significantly decreased migration of cells into the scratched wound area. In contrast, A4GALT suppression improved tube formation and cell migration capacity. Western blot analysis revealed that MAPK and AKT phosphorylation levels were downregulated while SOD and catalase were upregulated in GLA-KO hiPSC-ECs. However, suppression of A4GALT restored these protein alterations. RNA sequencing analysis demonstrated significant transcriptome changes in GLA-mutant EC, especially in angiogenesis, cell death, and cellular response to oxidative stress. However, these were effectively restored in GLA/A4GALT-KO hiPSC-ECs.
CRISPR/Cas9-mediated A4GALT suppression rescued FD phenotype and endothelial dysfunction in GLA-mutant hiPSC-ECs, presenting a potential therapeutic approach for FD-vasculopathy.
Copyright © 2024 Elsevier B.V. All rights reserved.
Product Citations: 12
In Atherosclerosis on 1 October 2024 by Shin, Y. J., Chae, S. Y., et al.
-
Stem Cells and Developmental Biology
Preprint on Research Square on 12 September 2023 by Shin, Y. J., Chae, S. Y., et al.
Backgrounds: The objective of this study was to investigate the efficacy of CRISPR/Cas9-mediated A4GALT suppression in rescuing endothelial dysfunction in Fabry disease (FD) endothelial cells (FD-ECs) derived from human induced pluripotent stem cells (hiPSCs). Methods: We differentiated hiPSCs (WT (wild-type), WTC-11), GLA -mutant hiPSCs ( GLA- KO, CMC-Fb-002), and CRISPR/Cas9-mediated A4GALT -KO hiPSCs ( GLA/A4GALT- KO, Fb-002- A4GALT -KO) into ECs and compared FD phenotypes and endothelial dysfunction. We also analyzed the effect of A4GALT suppression on reactive oxygen species (ROS) formation and transcriptome profiles through RNA sequencing. Results: GLA -mutant hiPSC-ECs ( GLA -KO and CMC-Fb-002) showed downregulated expression of EC markers and significantly reduced α-GalA expression with increased Gb-3 deposition and intra-lysosomal inclusion bodies. However, CRISPR/Cas9-mediated A4GALT suppression in GLA/A4GALT -KO and Fb-002- A4GALT -KO hiPSC-ECs increased expression levels of EC markers and rescued these FD phenotypes. GLA -mutant hiPSC-ECs failed to form tube-like structure in tube formation assays with, showing significantly decreased migration of cells into the scratched wound area. In contrast, A4GALT suppression improved tube formation and cell migration capacity. Western blot analysis revealed that MAPK and AKT phosphorylation levels were downregulated whereas SOD and catalase were upregulated in GLA -KO hiPSC-ECs. However, suppression of A4GALT restored these protein alterations. RNA sequencing analysis demonstrated significant transcriptome changes in GLA -mutant EC, especially in angiogenesis, cell death, and cellular response to oxidative stress. However, these were effectively restored in GLA/A4GALT- KO hiPSC-ECs. Conclusions: CRISPR/Cas9-mediated A4GALT suppression rescued FD phenotype and endothelial dysfunction in GLA -mutant hiPSC-ECs, presenting a potential therapeutic approach for FD-vasculopathy.
-
Stem Cells and Developmental Biology
In Journal of Translational Medicine on 22 February 2023 by Cui, S., Fang, X., et al.
To explore the possibility of kidney organoids generated using patient derived human induced pluripotent stem cells (hiPSC) for modeling of Fabry disease nephropathy (FDN).
First, we generated hiPSC line using peripheral blood mononuclear cells (PBMCs) from two male FD-patients with different types of GLA mutation: a classic type mutation (CMC-Fb-001) and a non-classic type (CMC-Fb-003) mutation. Second, we generated kidney organoids using wild-type (WT) hiPSC (WTC-11) and mutant hiPSCs (CMC-Fb-001 and CMC-Fb-003). We then compared alpha-galactosidase A (α-GalA) activity, deposition of globotriaosylceremide (Gb-3), and zebra body formation under electromicroscopy (EM).
Both FD patients derived hiPSCs had the same mutations as those detected in PBMCs of patients, showing typical pluripotency markers, normal karyotyping, and successful tri-lineage differentiation. Kidney organoids generated using WT-hiPSC and both FD patients derived hiPSCs expressed typical nephron markers without structural deformity. Activity of α-GalA was decreased and deposition of Gb-3 was increased in FD patients derived hiPSCs and kidney organoids in comparison with WT, with such changes being far more significant in CMC-Fb-001 than in CMC-Fb-003. In EM finding, multi-lammelated inclusion body was detected in both CMC-Fb-001 and CMC-Fb-003 kidney organoids, but not in WT.
Kidney organoids generated using hiPSCs from male FD patients might recapitulate the disease phenotype and represent the severity of FD according to the GLA mutation type.
© 2023. The Author(s).
-
Homo sapiens (Human)
-
Stem Cells and Developmental Biology
Preprint on Research Square on 18 November 2022 by Cui, S., Fang, X., et al.
Objectives: To explore the possibility of kidney organoids generated using patient derived human induced pluripotent stem cells (hiPSC) for modeling of Fabry disease nephropathy (FDN). Methods: : First, we generated hiPSC line using peripheral blood mononuclear cells (PBMCs) from two male FD-patients with different types of GLA mutation: a classic type mutation (CMC-Fb-001) and a late-onset variant (CMC-Fb-003) mutation. Second, we generated kidney organoids using wild-type (WT) hiPSC (WTC-11) and mutant hiPSCs (CMC-Fb-001 and CMC-Fb-003). We then compared alpha-galactosidase A (α-GalA) activity, deposition of globotriaosylceremide (Gb-3), and zebra body formation under electromicroscopy (EM). Results: : Both FD patients derived hiPSCs had the same mutations as those detected in PBMCs of patients, showing typical pluripotency markers, normal karyotyping, and successful tri-lineage differentiation. Kidney organoids generated using WT-hiPSC and both FD patients derived hiPSCs expressed typical nephron markers without structural deformity. Activity of α-GalA was decreased and deposition of Gb-3 was increased in FD patients derived hiPSCs and kidney organoids in comparison with WT, with such changes being far more significant in CMC-Fb-001 than in CMC-Fb-003. In EM finding, multi-lammelated inclusion body was detected in both CMC-Fb-001 and CMC-Fb-003 kidney organoids, but not in WT. Conclusions: : Kidney organoids generated using hiPSCs from male FD patients might recapitulate the disease phenotype and represent the severity of FD according to the GLA mutation type.
-
Homo sapiens (Human)
-
Stem Cells and Developmental Biology
In FASEB BioAdvances on 1 November 2020 by Roy, K. R., Uddin, M. B., et al.
Glucosylceramide synthase (GCS) is a key enzyme catalyzing ceramide glycosylation to generate glucosylceramide (GlcCer), which in turn serves as the precursor for cells to produce glycosphingolipids (GSLs). In cell membranes, GSLs serve as essential components of GSL-enriched microdomains (GEMs) and mediate membrane functions and cell behaviors. Previous studies showed that ceramide glycosylation correlates with upregulated expression of p53 hotspot mutant R273H and cancer drug resistance. Yet, the underlying mechanisms remain elusive. We report herewith that globotriaosylceramide (Gb3) is associated with cSrc kinase in GEMs and plays a crucial role in modulating expression of p53 R273H mutant and drug resistance. Colon cancer cell lines, either WiDr homozygous for missense-mutated TP53 (R273H+/+) or SW48/TP53-Dox bearing heterozygous TP53 mutant (R273H/+), display drug resistance with increased ceramide glycosylation. Inhibition of GCS with Genz-161 (GENZ 667161) resensitized cells to apoptosis in these p53 mutant-carrying cancer cells. Genz-161 effectively inhibited GCS activity, and substantially suppressed the elevated Gb3 levels seen in GEMs of p53-mutant cells exposed to doxorubicin. Complex formation between Gb3 and cSrc in GEMs to activate β-catenin was detected in both cultured cells and xenograft tumors. Suppression of ceramide glycosylation significantly decreased Gb3-cSrc in GEMs, β-catenin, and methyltransferase-like 3 for m6A RNA methylation, thus altering pre-mRNA splicing, resulting in upregulated expression of wild-type p53 protein, but not mutants, in cells carrying p53 R273H. Altogether, increased Gb3-cSrc complex in GEMs of membranes in response to anticancer drug induced cell stress promotes expression of p53 mutant proteins and accordant cancer drug resistance.
©2020 The Authors. FASEB BioAdvances published by The Federation of American Societies for Experimental Biology.
-
Cancer Research
-
Genetics
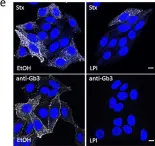
In Sci Rep on 26 July 2016 by Ailte, I., Lingelem, A. B., et al.
Fig.6.E

-
ICC-IF
-
Homo sapiens (Human)
Collected and cropped from Sci Rep by CiteAb, provided under a CC-BY license
Image 1 of 1