Hepatic fibrosis, ultimately causing hepatic sclerosis, remains significant health concerns. Adipose-derived mesenchymal stem cell (ADMSC)-derived exosomes (Exo) exhibit amelioration of liver injury. Hepatocyte growth factor (HGF) regulates hepatocyte growthn. However, its involvement during hepatic fibrosis remains unclear.
Isolation of ADMSCs and Exo, transfection of HGF overexpression, and activation of hepatic stellate cells (HSCs) by Angiotensin II (AngII) were conducted. Cells were randomized into HSC, AngII-HSC, ADMSCs-Exo, ADMSCsblank-Exo, and ADMSCsHGF-Exo, DPI, LY294002, and SB203580 groups. MTT for cell viability, cell migration, and flow cytometry for ROS were performed. BALB/c mice were treated with CCL4 for hepatic fibrosis models. The mice were randomized into Control, PBS, ADMSCs-Exo, ADMSCsblank-Exo, and ADMSCsHGF-Exo groups (n=6). HE, Sirius red, and Oil Red O staining, liver function indicators, and ELISA for oxidative stress were performed. ROS generation-related and PI3K/Akt/P38MAPK-related factors were detected by immunofluorescence, immunohistochemistry, and western blot.
After identification of ADMSC-Exo and transfection, AngII increased cell viability, migration, Collagen I (CoLI), α-smooth muscle actin (α-SMA), ROS, NADPH oxidase 4 (NOX4), PI3K, p-Akt, p-P38MAPK, ras-related C3 botulinum toxin substrate 1 (RAC1), p47phox, and p22phox expression. However, ADMSCsHGF-Exo, DPI, LY294002, and SB203580 reversed the above effects. Moreover, ADMSCsHGF-Exo inhibited pathological damage, fibrosis, lipid accumulation, ALT, AST, TBIL, CoLI, α-SMA, NOX4, MDA, PI3K, P-Akt, and P-P38MAPK expression, and increased ALB, SOD, GPx, CAT, GSH, Mn-SOD, Na+-K+-ATPase, and Ca2+-Mg2+-ATPase levels in hepatic fibrosis mice.
ADMSCsHGF-Exo attenuated hepatic fibrosis by inhibiting oxidative stress through activating the PI3K/Akt/P38MAPK pathway, providing valuable insights for potential treatment of liver fibrosis.
©The Author(s) 2024. Open Access. This article is licensed under a Creative Commons CC-BY International License.
Product Citations: 74
In Histology and Histopathology on 1 May 2025 by Zhou, H., Wu, Y., et al.
-
Pathology
In Journal of Prosthodontic Research on 11 February 2025 by Kobayashi, T., Hata, M., et al.
Spark-discharged anodic oxidation coating on commercially pure titanium (SAc.p.Ti) has been shown to promote bone conduction and bone matrix mineralization during new bone formation. This study hypothesized that the combination of SAc.p.Ti with dental pulp stem cells (DPSCs) would enhance new bone formation. The objective was to evaluate the effect of this combination in a rat bone defect model.
DPSCs were isolated from Sprague-Dawley (SD) rat incisors and cultured. Calvarial bone defects were created in SD rats, followed by transplantation of commercially pure titanium (c.p.Ti), SAc.p.Ti, or SAc.p.Ti combined with DPSCs. Bone formation was assessed using micro-computed tomography (micro-CT). Toluidine blue O staining was employed to evaluate bone-implant contact and the newly formed bone area. Hematoxylin-eosin (HE) staining was performed to identify osteoblast-like cells.
Micro-CT analysis revealed hard tissue formation on the surface of SAc.p.Ti. Toluidine blue O staining showed significantly greater bone-implant contact and newly formed bone area in the SAc.p.Ti/DPSC group compared to the c.p.Ti and SAc.p.Ti groups. HE staining confirmed the presence of osteoblast-like cells at the defect margins, with evidence of new bone formation on the surface of SAc.p.Ti and in the SAc.p.Ti/DPSC groups.
The combination of SAc.p.Ti and DPSCs presents a promising strategy for promoting new bone formation in rat calvarial defect model.
-
Stem Cells and Developmental Biology
A glucocorticoid spike derails muscle repair to heterotopic ossification after spinal cord injury.
In Cell Reports Medicine on 17 December 2024 by Alexander, K. A., Tseng, H. W., et al.
Why severe injury to the central nervous system (CNS) triggers the development of large neurogenic heterotopic ossifications (NHOs) within periarticular muscles remains unknown. We report that spinal cord injury (SCI) triggers a rapid corticosterone spike in mice, which is causal for NHO development because treatments with corticosterone or the synthetic glucocorticoid (GC) receptor (GR) agonist dexamethasone are sufficient to trigger heterotopic ossification and upregulate the expression of osteoinductive and osteogenic differentiation genes in injured muscles even without SCI. The central role for GR signaling in causing NHO is further demonstrated in mice deleted for the GR gene (Nr3c1), which no longer develop NHO after SCI. Furthermore, administration of clinical GR antagonists inhibits NHO development in mice with SCI. This study identifies endogenous GC as causing pathological NHO after CNS injury and suggests that GR antagonists may be of prophylactic use to prevent NHO development in victims of severe CNS injuries.
Copyright © 2024 The Authors. Published by Elsevier Inc. All rights reserved.
-
Neuroscience
In Journal of Cachexia, Sarcopenia and Muscle on 1 October 2024 by Li, F., Zhang, F., et al.
Aging negatively impacts tissue repair, particularly in skeletal muscle, where the regenerative capacity of muscle stem cells (MuSCs) diminishes with age. Although aerobic exercise is known to attenuate skeletal muscle atrophy, its specific impact on the regenerative and repair capacity of MuSCs remains unclear.
Mice underwent moderate-intensity continuous training (MICT) from 9 months (aged + Ex-9M) or 20 months (aged + Ex-20M) to 25 months, with age-matched (aged) and adult controls. Histological examinations and MuSC transplantation assays assessed aerobic exercise effects on MuSC function and muscle regeneration. CCN2/connective tissue growth factor modulation (overexpression and knockdown) in MuSCs and AICAR supplementation effects were explored.
Aged mice displayed significantly reduced running duration (65.33 ± 4.32 vs. 161.9 ± 1.29 min, mean ± SD, P < 0.001) and distance (659.17 ± 103.64 vs. 3058.28 ± 46.26 m, P < 0.001) compared with adults. This reduction was accompanied by skeletal muscle weight loss and decreased myofiber cross-sectional area (CSA). However, MICT initiated at 9 or 20 months led to a marked increase in running duration (142.75 ± 3.14 and 133.86 ± 20.47 min, respectively, P < 0.001 compared with aged mice) and distance (2347.58 ± 145.11 and 2263 ± 643.87 m, respectively, P < 0.001). Additionally, MICT resulted in increased skeletal muscle weight and enhanced CSA. In a muscle injury model, aged mice exhibited fewer central nuclear fibres (CNFs; 266.35 ± 68.66/mm2), while adult, aged + Ex-9M and aged + Ex-20M groups showed significantly higher CNF counts (610.82 ± 46.76, 513.42 ± 47.19 and 548.29 ± 71.82/mm2, respectively; P < 0.001 compared with aged mice). MuSCs isolated from aged mice displayed increased CCN2 expression, which was effectively suppressed by MICT. Transplantation of MuSCs overexpressing CCN2 (Lenti-CCN2, Lenti-CON as control) into injured tibialis anterior muscle compromised regeneration capacity, resulting in significantly fewer CNFs in the Lenti-CCN2 group compared with Lenti-CON (488.07 ± 27.63 vs. 173.99 ± 14.28/mm2, P < 0.001) at 7 days post-injury (dpi). Conversely, knockdown of CCN2 (Lenti-CCN2shR, Lenti-NegsiR as control) in aged MuSCs improved regeneration capacity, significantly increasing the CNF count from 254.5 ± 26.36 to 560.39 ± 48.71/mm2. Lenti-CCN2 MuSCs also increased fibroblast proliferation and exacerbated skeletal muscle fibrosis, while knockdown of CCN2 in aged MuSCs mitigated this pattern. AICAR supplementation, mimicking exercise, replicated the beneficial effects of aerobic exercise by mitigating muscle weight decline, enhancing satellite cell activity and reducing fibrosis.
Aerobic exercise effectively reverses the decline in endurance capacity and mitigates muscle atrophy in aged mice. It inhibits CCN2 secretion from senescent MuSCs, thereby enhancing skeletal muscle regeneration and preventing fibrosis in aged mice. AICAR supplementation mimics the beneficial effects of aerobic exercise.
© 2024 The Author(s). Journal of Cachexia, Sarcopenia and Muscle published by Wiley Periodicals LLC.
-
Stem Cells and Developmental Biology
Zinc-alpha-2-glycoprotein Secreted by Triple-Negative Breast Cancer Promotes Peritumoral Fibrosis.
In Cancer Res Commun on 1 July 2024 by Verma, S., Giagnocavo, S. D., et al.
Obesity is a modifiable predisposition factor for postmenopausal breast cancer. This suggests a localized, reciprocal interaction between breast cancer cells and the surrounding mammary white adipose tissue. To investigate how breast cancer cells alter the composition and function of adipose tissue, we screened the secretomes of 10 human breast cancer cell lines for the ability to modulate the differentiation of adipocyte stem and progenitor cells. The screen identified an adipogenic modulator, zinc-alpha-2-glycoprotein (ZAG/AZGP1) that is secreted by triple-negative breast cancer (TNBC) cells. TNBC-secreted ZAG inhibits adipogenesis and instead induces the expression of fibrotic genes. Accordingly, depletion of ZAG in TNBC cells attenuates fibrosis in white adipose tissue and inhibits tumor growth. Further, high expression of ZAG is linked to poor prognosis in patients with TNBC but not in patients with other clinical subtypes of breast cancer. Our findings suggest a role of TNBC-secreted ZAG in promoting the transdifferentiation of adipocyte stem and progenitor cells into cancer-associated fibroblasts to support tumorigenesis.
Functional screening of breast cancer secretomes revealed that triple-negative breast cancer promotes fibrosis in the adipose tissue microenvironment by secreting zinc-alpha-2-glycoprotein and promoting the transdifferentiation of adipocyte stem cells into myofibroblasts.
©2024 The Authors; Published by the American Association for Cancer Research.
-
Mus musculus (House mouse)
-
Cancer Research
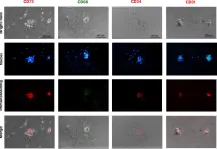
In Stem Cell Res Ther on 4 July 2023 by Moein, S., Ahmadbeigi, N., et al.
Fig.3.A

-
ICC-IF
-
Mus musculus (House mouse)
Collected and cropped from Stem Cell Res Ther by CiteAb, provided under a CC-BY license
Image 1 of 3
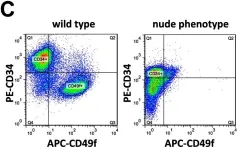
In PLoS One on 23 May 2013 by Bohr, S., Patel, S. J., et al.
Fig.6.C

-
FC/FACS
-
Mus musculus (House mouse)
Collected and cropped from PLoS One by CiteAb, provided under a CC-BY license
Image 1 of 3
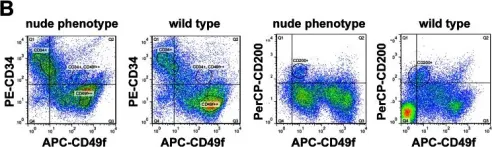
In PLoS One on 23 May 2013 by Bohr, S., Patel, S. J., et al.
Fig.4.B

-
FC/FACS
-
Mus musculus (House mouse)
Collected and cropped from PLoS One by CiteAb, provided under a CC-BY license
Image 1 of 3