Double C2-like domain beta (DOC2B) is a vesicle priming protein critical for glucose-stimulated insulin secretion in β-cells. Individuals with type 1 diabetes (T1D) have lower levels of DOC2B in their residual functional β-cell mass and platelets, a phenotype also observed in a mouse model of T1D. Thus, DOC2B levels could provide important information on β-cell dys(function).
Our objective was to evaluate the DOC2B secretome of β-cells. In addition to soluble extracellular protein, we assessed DOC2B localized within membrane-delimited nanoparticles - extracellular vesicles (EVs). Moreover, in rat clonal β-cells, we probed domains required for DOC2B sorting into EVs.
Using Single Extracellular VEsicle Nanoscopy, we quantified EVs derived from clonal β-cells (human EndoC-βH1, rat INS-1 832/13, and mouse MIN6); two other cell types known to regulate glucose homeostasis and functionally utilize DOC2B (skeletal muscle rat myotube L6-GLUT4myc and human neuronal-like SH-SY5Y cells); and human islets sourced from individuals with no diabetes (ND). EVs derived from ND human plasma, ND human islets, and cell lines were isolated with either size exclusion chromatography or differential centrifugation. Isolated EVs were comprehensively characterized using dotblots, transmission electron microscopy, nanoparticle tracking analysis, and immunoblotting.
DOC2B was present within EVs derived from ND human plasma, ND human islets, and INS-1 832/13 β-cells. Compared to neuronal-like SH-SY5Y cells and L6-GLUT4myc myotubes, clonal β-cells (EndoC-βH1, INS-1 832/13, and MIN6) produced significantly more EVs. DOC2B levels in EVs (over whole cell lysates) were higher in INS-1 832/13 β-cells compared to L6-GLUT4myc myotubes; SH-SY5Y neuronal-like cells did not release appreciable DOC2B. Mechanistically, we show that DOC2B was localized to the EV lumen; the tandem C2 domains were sufficient to confer sorting to INS-1 832/13 β-cell EVs.
Clonal β-cells and ND human islets produce abundant EVs. In cell culture, appreciable DOC2B can be packaged into EVs, and a small fraction is excreted as a soluble protein. While DOC2B-laden EVs and soluble protein are present in ND plasma, further studies will be necessary to determine if DOC2B originating from β-cells significantly contributes to the plasma secretome.
Copyright © 2024 Esparza, Lima, Abuelreich, Ghaeli, Hwang, Oh, Lenz, Gu, Jiang, Kandeel, Thurmond and Jovanovic-Talisman.
Product Citations: 14
In Frontiers in Endocrinology on 5 November 2024 by Esparza, D., Lima, C., et al.
-
Endocrinology and Physiology
In Journal of Extracellular Vesicles on 1 September 2023 by Solana-Balaguer, J., Campoy-Campos, G., et al.
Extracellular vesicles (EVs) play an important role in intercellular communication as carriers of signalling molecules such as bioactive miRNAs, proteins and lipids. EVs are key players in the functioning of the central nervous system (CNS) by influencing synaptic events and modulating recipient neurons. However, the specific role of neuron-to-neuron communication via EVs is still not well understood. Here, we provide evidence that primary neurons uptake neuron-derived EVs in the soma, dendrites, and even in the dendritic spines, and carry synaptic proteins. Neuron-derived EVs increased spine density and promoted the phosphorylation of Akt and ribosomal protein S6 (RPS6), via TrkB-signalling, without impairing the neuronal network activity. Strikingly, EVs exerted a trophic effect on challenged nutrient-deprived neurons. Altogether, our results place EVs in the spotlight for synaptic plasticity modulation as well as a possible therapeutic tool to fight neurodegeneration.
© 2023 The Authors. Journal of Extracellular Vesicles published by Wiley Periodicals LLC on behalf of International Society for Extracellular Vesicles.
-
FC/FACS
-
Rattus norvegicus (Rat)
-
Neuroscience
Circulating Pro-Inflammatory Exosomes Worsen Stroke Outcomes in Aging.
In Circulation Research on 17 September 2021 by Zhang, H., Lin, S., et al.
In Journal of Cell Science on 1 May 2021 by Freitas Filho, E. G., da Silva, E. Z. M., et al.
Although RACK1 is known to act as a signaling hub in immune cells, its presence and role in mast cells (MCs) is undetermined. MC activation via antigen stimulation results in mediator release and is preceded by cytoskeleton reorganization and Ca2+ mobilization. In this study, we found that RACK1 was distributed throughout the MC cytoplasm both in vivo and in vitro. After RACK1 knockdown (KD), MCs were rounded, and the cortical F-actin was fragmented. Following antigen stimulation, in RACK1 KD MCs, there was a reduction in cortical F-actin, an increase in monomeric G-actin and a failure to organize F-actin. RACK1 KD also increased and accelerated degranulation. CD63+ secretory granules were localized in F-actin-free cortical regions in non-stimulated RACK1 KD MCs. Additionally, RACK1 KD increased antigen-stimulated Ca2+ mobilization, but attenuated antigen-stimulated depletion of ER Ca2+ stores and thapsigargin-induced Ca2+ entry. Following MC activation there was also an increase in interaction of RACK1 with Orai1 Ca2+-channels, β-actin and the actin-binding proteins vinculin and MyoVa. These results show that RACK1 is a critical regulator of actin dynamics, affecting mediator secretion and Ca2+ signaling in MCs. This article has an associated First Person interview with the first author of the paper.
© 2021. Published by The Company of Biologists Ltd.
-
Rattus norvegicus (Rat)
-
Cell Biology
Inhibitory role of Munc13-1 in antigen-induced mast cell degranulation.
In Biomedical Research (Tokyo, Japan) on 12 December 2017 by Higashio, H., Satoh, Y. I., et al.
Secretory granules (SGs) of mast cells are lysosome-related organelles that contain various inflammatory molecules such as histamine, which are stored in the cytoplasm. Mast cell degranulation is the regulated exocytosis of SGs in response to external stimuli, such as the antigen-mediated cross-linking of the high-affinity IgE receptor, FcεRI. Upon stimulation, SGs undergo priming to become fusion-competent prior to fusing with the plasma membrane, which is mediated by Munc13-4, one of the five members of the vesicle-priming Munc13 protein family. Although Munc13-4 is shown to be crucial for mast cell degranulation, the functional involvement of other Munc13 isoform(s) remains unknown. Herein, this was investigated using the RBL-2H3 mast cell line. We found that Munc13-1 and Munc13-4 are the only Munc13 isoforms that are expressed in the RBL-2H3 cells, and Munc13-1 is distributed in the cytoplasm, but highly concentrated on the late endosome and/or lysosome. Unexpectedly, antigen-induced degranulation was considerably increased by Munc13-1 knockdown, but decreased by its overexpression. Further, we found that the hypersecretion phenotype of the Munc13-1-knockdown cells was attenuated by simultaneous Munc13-4 knockdown. These results suggested that Munc13-1 has an inhibitory role in antigen-induced mast cell degranulation, which is performed in a Munc13-4-dependent manner.
-
Immunology and Microbiology
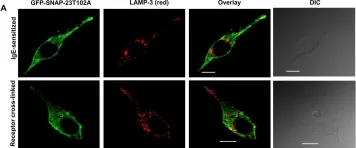
In Biol Open on 15 September 2017 by Naskar, P. & Puri, N.
Fig.5.A

-
ICC-IF
-
Rattus norvegicus (Rat)
Collected and cropped from Biol Open by CiteAb, provided under a CC-BY license
Image 1 of 2
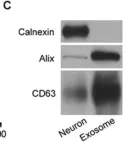
In Cell Res on 1 July 2017 by Xu, B., Zhang, Y., et al.
Fig.4.C

-
WB
-
Collected and cropped from Cell Res by CiteAb, provided under a CC-BY license
Image 1 of 2