Virulence factors are required for bacterial pathogens to establish infection; however, their expression can be energetically costly and must be tightly controlled to avoid fitness costs. Expression can be controlled at specific stages during infection (temporal regulation) or expressed by small subsets of the bacterial population (spatial regulation). There has been a great deal of interest in developing virulence factor-targeting strategies to combat Staphylococcus aureus infection, but the spatiotemporal regulation of the virulence factor master regulatory systems (Agr, Sae) has not been explored during kidney abscess formation. This information is critical for the design of therapeutics targeting these pathways. Here, we utilized a fluorescent transcriptional reporter approach to visualize dynamics in Agr and Sae activity during abscess formation in the mouse kidney. We categorized kidney abscess formation into four stages, then defined spatiotemporal gene expression. Agr signaling appeared inactive in the kidney; consistent with this, agr mutant abscesses fully developed. In contrast, we observed heterogeneous (ON/OFF) activity of Sae at early stages, where bacteria were found intracellularly within neutrophils. Sae activity increased as abscesses developed, and heterogeneity in spatial patterning was observed, but patterns varied between abscesses, suggesting distinct microenvironments within individual abscesses. Consistent with a requirement for Sae activity during abscess development, the sae mutant did not develop abscesses past early stages. These results have implications for the genes regulated by Agr and Sae and suggest a requirement for Sae activity during kidney abscess development.
Infections with Staphylococcus aureus pose a serious public health threat due to high levels of antibiotic resistance and limited efficacy of alternative therapeutics. There has been a great deal of interest in developing novel therapeutics that target virulence factors essential during infection. However, it remains largely unknown if these factors are required at specific stages of the infection, and whether all bacterial cells or a limited subset express them. Here, we sought to examine virulence factor expression using fluorescent reporter strains that would indicate activity of two master regulators of virulence in S. aureus, Agr and Sae. While Agr appeared inactive during kidney abscess development, the Sae system exhibited heterogeneity, increased expression at later stages, and was required for abscess progression. These results provide critical information for the development of virulence factor-targeting strategies for kidney abscess treatment.
Product Citations: 463
In mBio on 10 December 2025 by Anil, A., Braza, R. E. D., et al.
-
Immunology and Microbiology
In Frontiers in Immunology on 5 December 2025 by Weissfuss, C., Hoffmann, K., et al.
The increasing prevalence of multidrug-resistant (MDR) bacteria has reduced the effectiveness of standard antibiotics, prompting renewed interest in bacteriophage (phage) therapy as an alternative or adjunctive treatment. Phage therapy offers high specificity, self-amplification at infection sites, and minimal disruption to the gut microbiota. However, clinical implementation is challenging, due to the risk of phage resistance and uncertainties regarding optimal dosing and immune interactions.
Previously, we demonstrated that a two-phage cocktail exhibited low immunogenicity in mice and, when combined with meropenem, significantly improved clearance of ventilator-associated Pseudomonas aeruginosa pneumonia, reduced inflammation, and disrupted biofilms more effectively than either treatment alone. In the present study, we investigated the interplay between this phage cocktail and innate immune defenses using a murine respiratory infection model and human in vitro assays.
Our findings reveal that the therapeutic efficacy of phage treatment is critically dependent on the presence of neutrophils, which act synergistically with phages to achieve effective bacterial clearance, particularly when bacterial burden exceeds a defined threshold. Alveolar macrophages, however, do not significantly contribute to infection resolution in vivo.
Since neutrophils play a key-role in supporting phage-mediated Pseudomonas clearance, the efficacy of phage therapy is closely linked to the hosts immune competence - an important consideration when treating immunocompromised patients.
Copyright © 2025 Weissfuss, Hoffmann, Behrendt, Bürkle, Twamley, Korf, Ahrens, Rohde, Zobel, Debarbieux, Ricard, Witzenrath and Nouailles.
-
Immunology and Microbiology
-
Cardiovascular biology
NEDD4L-mediated Gasdermin D and E ubiquitination regulates cell death and tissue injury.
In Cell Death and Differentiation on 19 November 2025 by Shah, S. S., Manning, J. A., et al.
The membrane pore-forming gasdermin (GSDM) proteins are essential executors of pyroptosis. The GSDM family members GSDMD and GSDME can also target mitochondrial membranes, driving apoptosis. Here, we identify the ubiquitin ligase NEDD4L as a key regulator of GSDMD and GSDME, two GSDMs involved in cell death. NEDD4L ubiquitinates both these proteins to control their stability and intracellular expression levels. Knockout of mouse Nedd4l (also called Nedd4-2) results in lung and kidney damage with perinatal lethality within three weeks of birth. These mice demonstrated elevated GSDMD in alveolar epithelia and increased GSDME in kidney tubular epithelia, suggesting tissue-specific regulation by NEDD4L. Renal tubule-specific Nedd4l knockout mice showed GSDM activation, tubular cell death and reduced kidney function after high sodium diet. NEDD4L-deficient cells showed increased GSDM activation, IL-1β release and were significantly more susceptible to cell death induced by NLRP3 agonists, cytotoxic agents, and bacterial infection. These results demonstrate that NEDD4L regulates GSDMD and GSDME functions by preventing their accumulation and reveals an unexplored link between GSDM stability and cell death.
© 2025. The Author(s).
-
Cell Biology
In Cells on 7 November 2025 by Zhang, M., Liu, N., et al.
Perineural invasion (PNI), defined by cancer spreading or invading into the nerve, links to severe pain, recurrence, and poor prognosis. PNI contributes to nerve damage, Schwann cell activation, and sensory neuron dysfunction. Soluble tumor necrosis factor α (solTNFα) binds to TNFR1 to drive inflammation and nerve injury, playing a key role in cancer progression and pain. This study, using a mouse sciatic nerve PNI model, explored whether blocking solTNFα-TNFR1 signaling via TNFR1 knockout or pharmacological inhibition by XPro1595 could reduce PNI-associated pain. Data showed that XPro1595, but not TNFR1 knockout, reduced tumor burden, alleviated mechanical allodynia, and improved muscle function and locomotion, primarily in females. Histological analysis in females showed that XPro1595 increased the number of myelin and dendritic cells while reducing axonal damage that resulted from PNI. In the tumor zone outside the nerve truck, XPro1595 reduced T cell and increased macrophage and dendritic cell numbers. Transcriptomic analysis revealed that XPro1595 in females with PNI upregulated mitochondrial, myelination, motor function, and immune regulation gene pathways while it downregulated inflammatory, extracellular matrix, and tumor progression pathways. Overall, we demonstrated that XPro1595 exhibited antitumor, neuroprotective, and analgesic properties in female mice, likely by promoting neuronal regeneration and mitochondrial function, while reducing inflammation and extracellular remodeling.
-
Cell Biology
Monoallelic mutations in MMD2 cause autosomal dominant aggressive periodontitis.
In The Journal of Experimental Medicine on 1 September 2025 by Iwata, T., Mizoguchi, Y., et al.
Aggressive periodontitis causes rapid destruction of periodontal tissue. It occurs at a young age with familial clustering. We report on the first time on molecular and cellular basis of a Mendelian form of autosomal dominant aggressive periodontitis. Monoallelic mutations in the monocyte to macrophage differentiation-associated 2 (MMD2) gene, encoding MMD2, in two Japanese families with autosomal dominant aggressive periodontitis are identified. Mutations, c.347 C>T (p.A116V) and c.377 G>C (p.R126P) in MMD2, disturbed fMLP-induced activation of Ras/ERK signaling. Additionally, abnormalities in the proteins of Golgi apparatus, a crucial contributor to innate immune signaling pathways, were identified in patients' neutrophils. The knock-in and knockout mice exhibited alveolar bone loss by ligature-induced periodontitis, along with impaired fMLP-induced chemotaxis, as found in the patients with MMD2 mutation. Our studies revealed that monoallelic mutations in MMD2 underlie the impairment of neutrophil chemotaxis, which leads to the development of autosomal dominant aggressive periodontitis.
© 2025 Iwata et al.
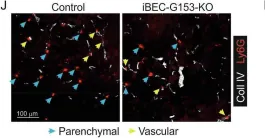
In Nat Commun on 7 July 2025 by Shao, J., Kwon, J., et al.
Fig.9.J
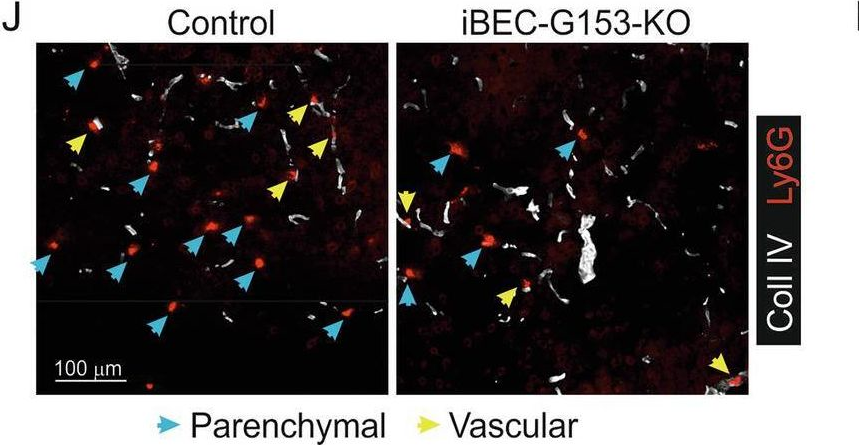
-
IHC-Fr-IF
-
Mus musculus (House mouse)
Collected and cropped from Nature Communications by CiteAb, provided under a CC-BY license
Image 1 of 18
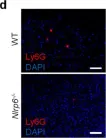
In Adv Sci (Weinh) on 1 February 2025 by Zhang, C., Zhang, X., et al.
Fig.7.A
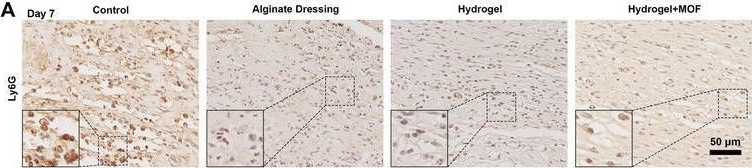
-
IHC
-
Collected and cropped from Advanced Science (Weinheim, Baden-Wurttemberg, Germany) by CiteAb, provided under a CC-BY license
Image 1 of 18
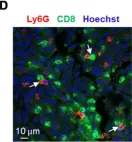
In Cells on 6 January 2022 by Mainz, R. E., Albers, S., et al.
Fig.4.D
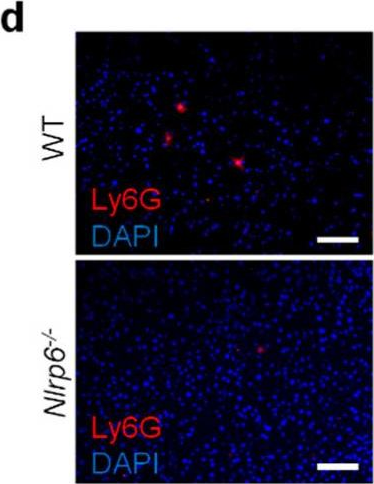
-
IHC-IF
-
Mus musculus (House mouse)
Collected and cropped from Cells by CiteAb, provided under a CC-BY license
Image 1 of 18
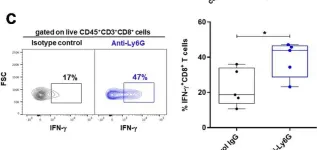
In Cancers (Basel) on 10 July 2020 by Khou, S., Popa, A., et al.
Fig.4.D
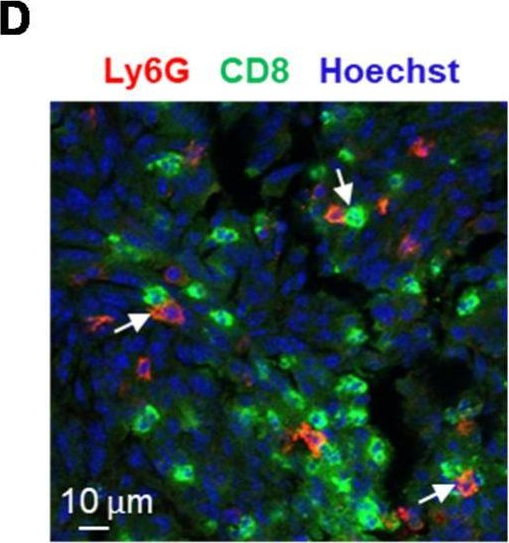
-
IHC-IF
-
Collected and cropped from Cancers by CiteAb, provided under a CC-BY license
Image 1 of 18
In Cancers (Basel) on 10 July 2020 by Khou, S., Popa, A., et al.
Fig.4.C
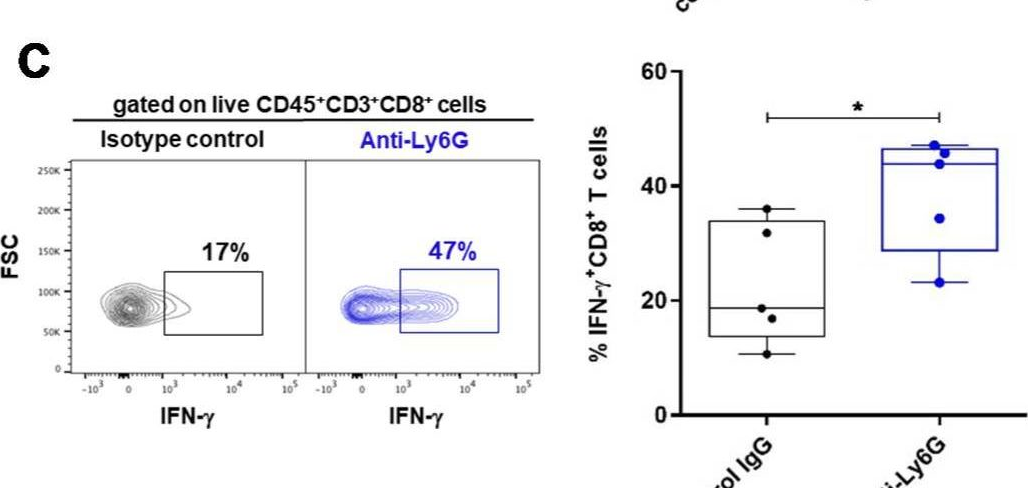
-
FC/FACS
-
Collected and cropped from Cancers by CiteAb, provided under a CC-BY license
Image 1 of 18
In Cancers (Basel) on 10 July 2020 by Khou, S., Popa, A., et al.
Fig.4.B
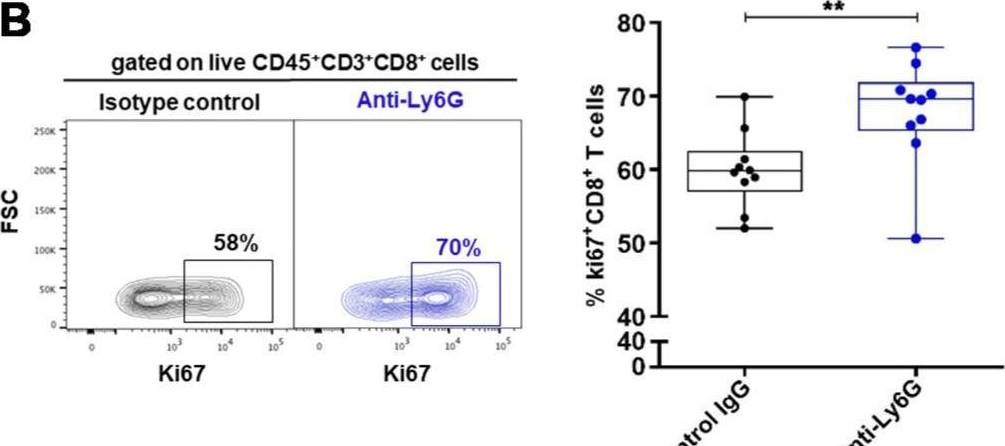
-
FC/FACS
-
Collected and cropped from Cancers by CiteAb, provided under a CC-BY license
Image 1 of 18
In Sci Rep on 26 November 2019 by Uyama, N., Tsutsui, H., et al.
Fig.3.A
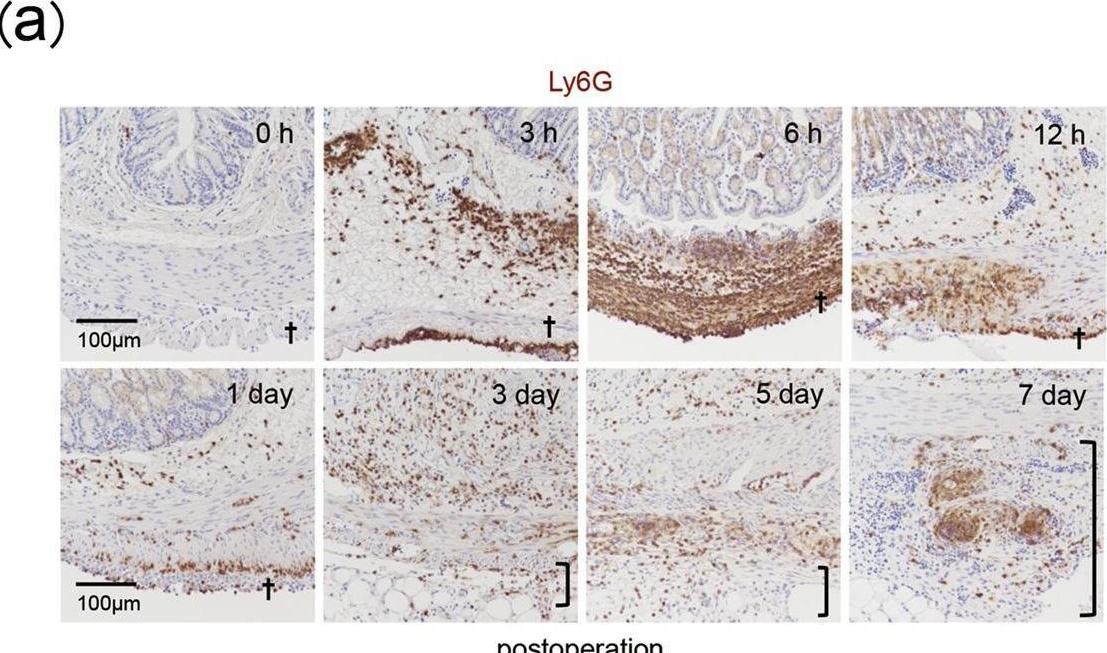
-
IHC
-
Collected and cropped from Scientific Reports by CiteAb, provided under a CC-BY license
Image 1 of 18
In Nat Commun on 18 April 2018 by Pircher, J., Czermak, T., et al.
Fig.7.I
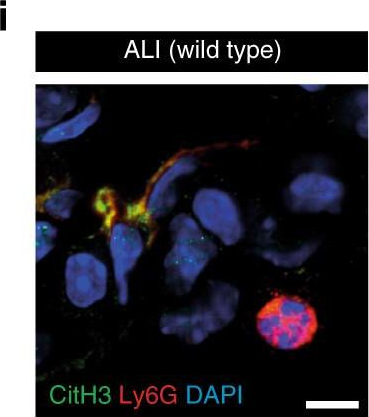
-
IHC-Fr-IF
-
Mus musculus (House mouse)
Collected and cropped from Nature Communications by CiteAb, provided under a CC-BY license
Image 1 of 18
In Vet Res on 7 November 2017 by Bouté, M., Carreras, F., et al.
Fig.5.C
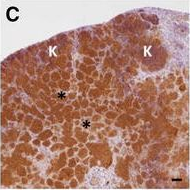
-
IHC
-
Bos taurus (Bovine)
Collected and cropped from Veterinary Research by CiteAb, provided under a CC-BY license
Image 1 of 18
In Vet Res on 7 November 2017 by Bouté, M., Carreras, F., et al.
Fig.4.D
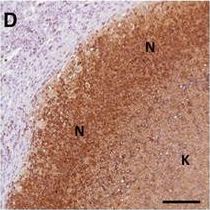
-
IHC
-
Bos taurus (Bovine)
Collected and cropped from Veterinary Research by CiteAb, provided under a CC-BY license
Image 1 of 18
In Vet Res on 7 November 2017 by Bouté, M., Carreras, F., et al.
Fig.4.C
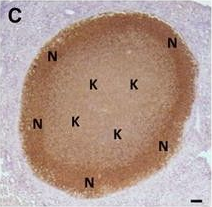
-
IHC
-
Bos taurus (Bovine)
Collected and cropped from Veterinary Research by CiteAb, provided under a CC-BY license
Image 1 of 18
In PLoS Genet on 1 March 2017 by Jin, Z., Liang, F., et al.
Fig.5.N
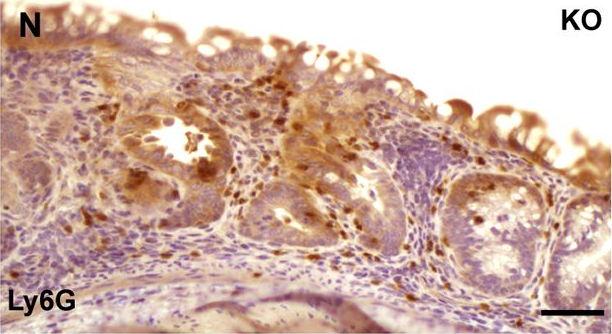
-
IHC
-
Mus musculus (House mouse)
Collected and cropped from PLoS Genetics by CiteAb, provided under a CC-BY license
Image 1 of 18
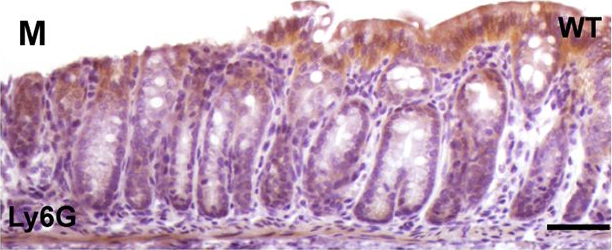
In PLoS Genet on 1 March 2017 by Jin, Z., Liang, F., et al.
Fig.5.M
-
IHC
-
Mus musculus (House mouse)
Collected and cropped from PLoS Genetics by CiteAb, provided under a CC-BY license
Image 1 of 18
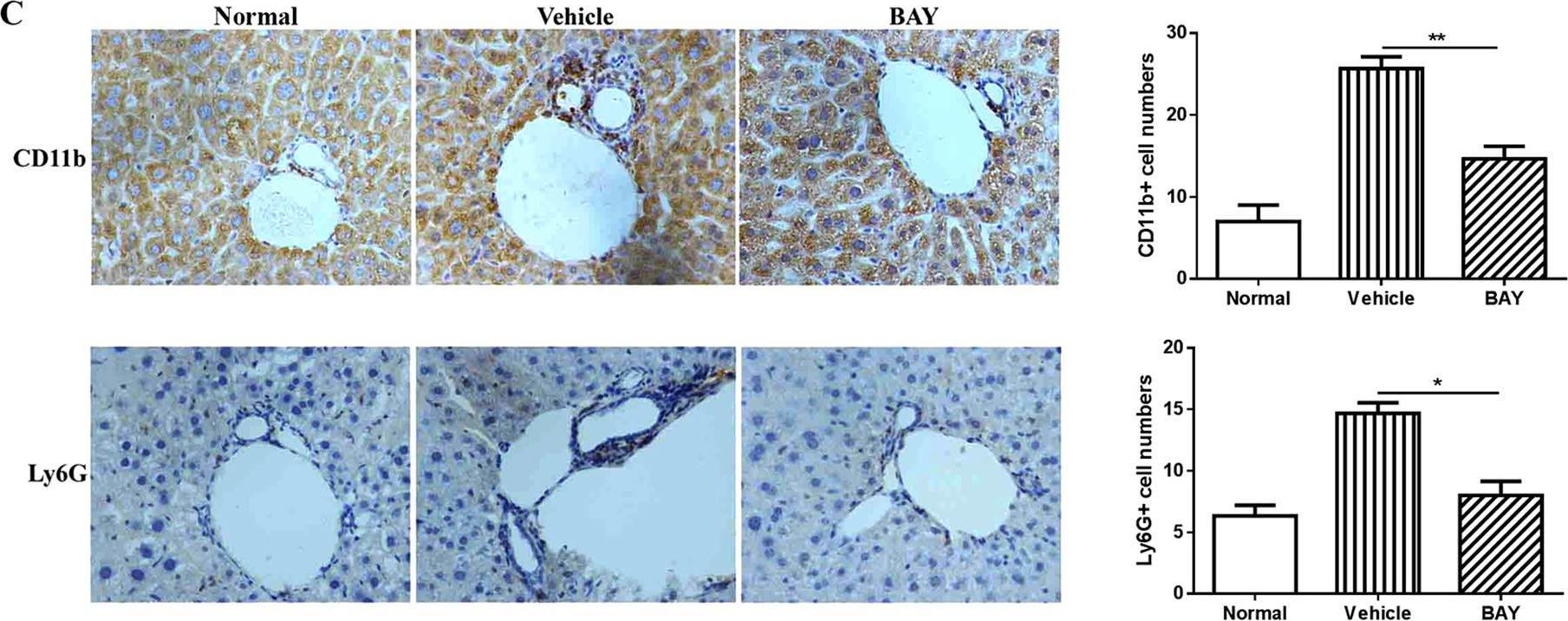
In Sci Rep on 4 December 2015 by Qiao, J., Huang, Y., et al.
Fig.5.C
-
IHC
-
Mus musculus (House mouse)
Collected and cropped from Scientific Reports by CiteAb, provided under a CC-BY license
Image 1 of 18
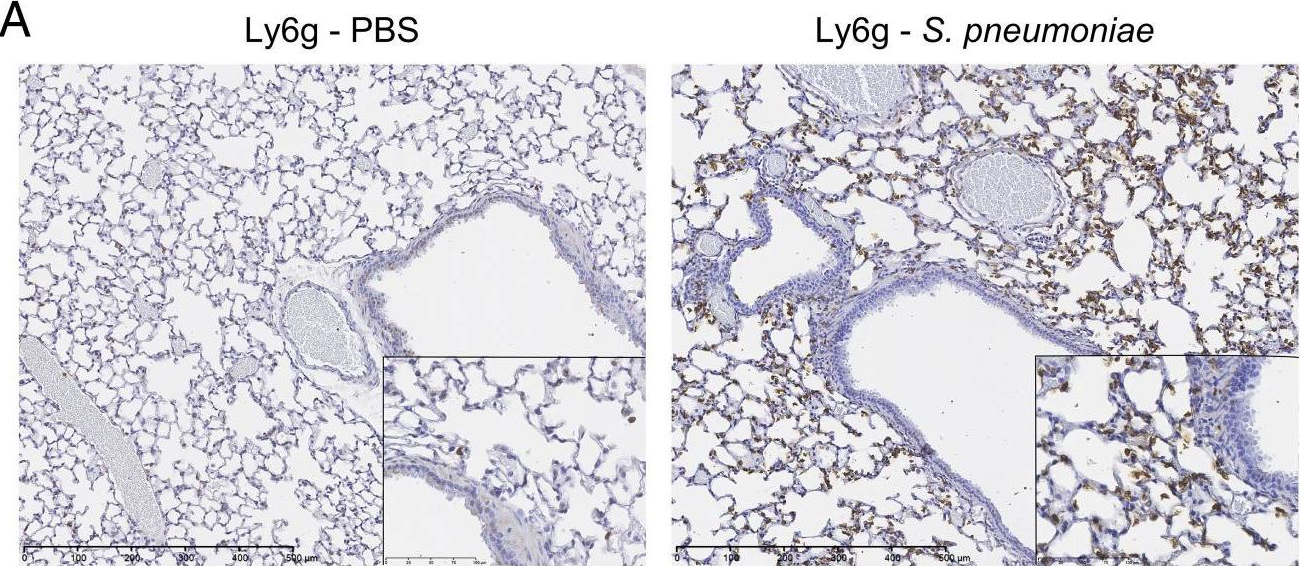
In J Immunol on 15 June 2015 by José, R. J., Williams, A. E., et al.
Fig.1.A
-
IHC-P
-
Mus musculus (House mouse)
Collected and cropped from The Journal of Immunology by CiteAb, provided under a CC-BY license
Image 1 of 18
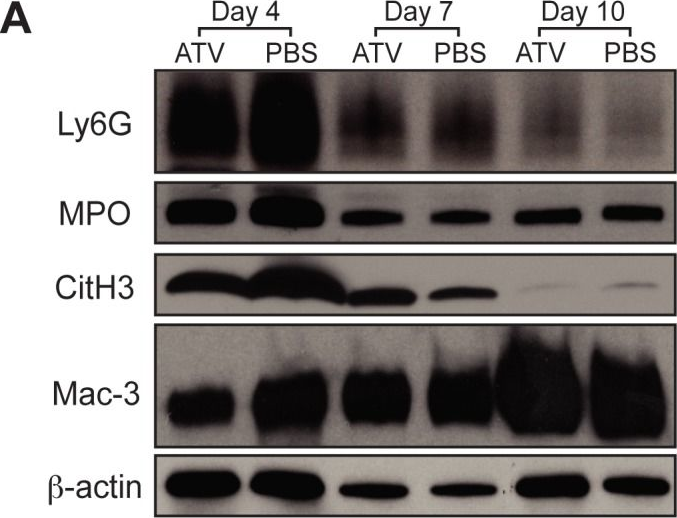
In PLoS One on 14 February 2015 by Kessinger, C. W., Kim, J. W., et al.
Fig.5.A
-
WB
-
Collected and cropped from PLoS ONE by CiteAb, provided under a CC-BY license
Image 1 of 18
In PLoS One on 1 June 2014 by Freeman, C. M., Quillin, R. C., et al.
Fig.1.D
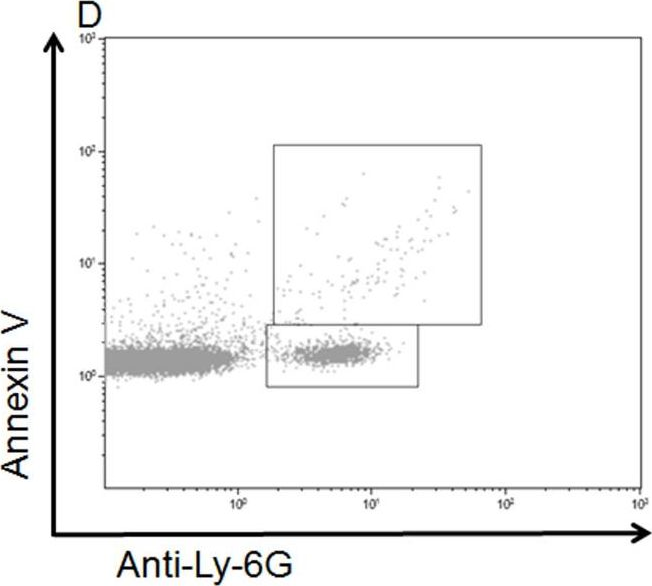
-
FC/FACS
-
Collected and cropped from PLoS ONE by CiteAb, provided under a CC-BY license
Image 1 of 18
In Mol Vis on 19 August 2011 by Liu, G., Lu, P., et al.
Fig.3.A
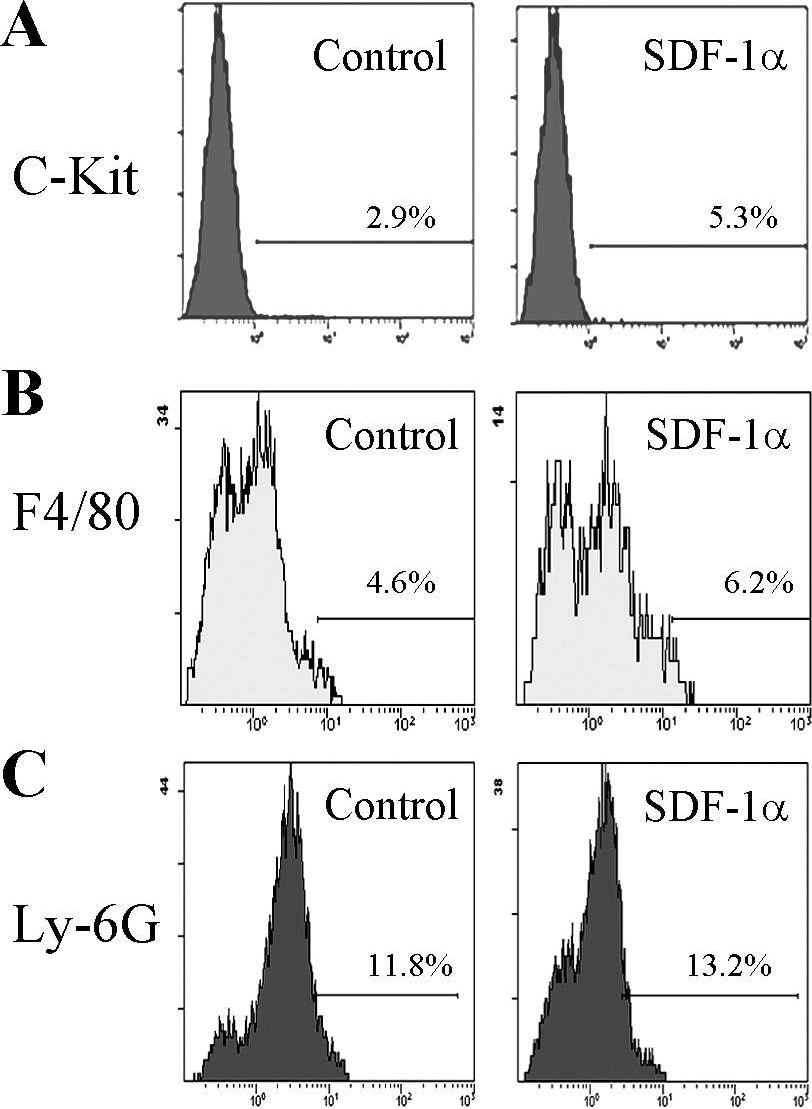
-
FC/FACS
-
Collected and cropped from Molecular Vision by CiteAb, provided under a CC-BY license
Image 1 of 18