The excessive accumulation of neutrophils within the epidermis is a significant hallmark of cutaneous diseases; however, the mechanisms governing neutrophil transepidermal migration (NTEM) remain inadequately understood. In this study, we develop trichromatic-fluorescence-labeled chimeric mice by utilizing Cx3cr1GFP/+Lyz2RFP/+ mice as bone marrow donors and Krt14YFP/+ mice as recipients. This approach enables us to visualize the process of NTEM and the crosstalk between neutrophils and monocytes in a murine model of irritant contact dermatitis (ICD). Intravital imaging reveals a preferential transmigration of neutrophils through hair follicle (HF), where dermal neutrophils exhibit limited mobility and interact with dermal monocytes. Notably, 18 h following hapten exposure, dermal neutrophils continuously migrate toward HF regions and form clusters within 3 h. Importantly, MMP-9 is identified as essential for the NTEM process; the depletion of dermal monocytes results in a significant reduction of MMP-9 expression in the skin and inhibits the NTEM process in ICD. Mechanistically, dermal monocytes are found to be a crucial source of the cytokines TNF-α and CXCL2, which promote the upregulation of MMP-9 in neutrophils. Therefore, our results highlight HF regions as crucial gateways for dermal monocyte-modulated NTEM and provide visual insights into the crosstalk between neutrophils and monocytes in inflammatory skin disorders.
© 2025. The Author(s).
Product Citations: 294
In Communications Biology on 4 April 2025 by Fan, Z., Xu, Y., et al.
-
FC/FACS
-
Mus musculus (House mouse)
Proton-secreting cells modulate mucosal immune surveillance in the male reproductive tract
Preprint on BioRxiv : the Preprint Server for Biology on 26 March 2025 by Da Silva, A., Barrachina, F., et al.
Proton-secreting cells in various organs, such as the kidney and epididymis, regulate pH balance, maintaining cellular homeostasis, and supporting key physiological processes. More recently, these specialized cells have emerged as key contributors to mucosal immunity, orchestrating immune activation. Epididymitis is an inflammatory condition that significantly impacts male fertility, often due to a lack of diagnosis and treatment. This study explores the involvement of region-specific epididymal proton-secreting clear cells (CCs) in the immune response by interacting with the immune system during LPS-induced mouse epididymitis. We found that in response to LPS, CCs rapidly shifted to a proinflammatory phenotype, marked by the upregulation of cytokines and chemokines, alongside the downregulation of genes involved in sperm maturation. Morphological changes in CCs, including increased apical blebs and altered shape across different epididymal segments, suggest their active role in immune responses. Moreover, mononuclear phagocytes (MPs) reduced their luminal-reaching projections in the proximal epididymis after the LPS challenge. This bacteria antigen triggered the migration of dendritic cells and neutrophil infiltration in the distal epididymis. These immune landscape alterations contributed to epithelial damage and impaired sperm maturation, as evidenced by decreased sperm motility following LPS injection. Our findings indicate that proton-secreting cells are immune gatekeepers in the epididymis, initiating immune responses and disrupting sperm maturation. This research enhances the understanding of epithelial immunoregulation and will help to develop novel diagnostic and therapeutic strategies for epididymitis and male infertility. Furthermore, insights into CC-mediated immune responses could inform the development of new approaches for male contraception.
-
Immunology and Microbiology
In Frontiers in Immunology on 18 March 2025 by Cai, Y., Yang, Q., et al.
Under hyperglycemic conditions, impaired intestinal barrier integrity leads to heightened level of inflammation, playing important roles in driving diabetic complications. Emerging evidence supports the implications of neutrophil extracellular traps (NETs) in the pathogenesis of diabetes. However, whether NETs contribute to hyperglycemia-linked intestinal barrier impairment remains to be investigated. Moreover, baicalin, the major chemical component of Scutellaria baicalensis Georgi, is equipped with twofold intestinal protective and neutrophil suppressive activities. Yet, it is unclear if baicalin is effective at mitigating hyperglycemia-linked NETs-mediated intestinal barrier impairment.
To directly address the mechanistic implications of NETs in hyperglycemia-linked intestinal epithelial barrier impairment, the impact of DNase I treatment or Padi4 gene deficiency on intestinal epithelial integrity was first examined in the streptozotocin (STZ)-induced hyperglycemic mice in vivo. Next, the pharmacological impact of baicalin on NETs formation and intestinal epithelial barrier impairment was investigated in high glucose- and/or lipopolysaccharides (LPS)-stimulated neutrophils in vitro and in STZ-induced hyperglycemic mice in vivo, respectively.
The in vitro experiments confirmed that high glucose and/or LPS induced NETs formation. NETs directly impaired the viability and tight junction of the intestinal epithelial cells. The histological and immunohistochemical examinations unveiled that along with impaired intestinal epithelial morphology, citrullinated histone H3 (H3Cit), a marker of NETs, and neutrophil specific Ly6G were readily detected in the intestinal epithelium in the hyperglycemic mice. Without affecting the presence of neutrophils, DNase I treatment or Padi4 gene deficiency markedly mitigated intestinal NETs formation and improved the intestinal morphology in the hyperglycemic mice. Notably, baicalin suppressed NETs formation and inhibited histone H3 citrullination stimulated by high glucose, LPS or both in vitro. Furthermore, baicalin blunted NETs formation and partially preserved the integrity of the intestinal epithelium in the hyperglycemic mice in vivo.
The current study sheds new light on the pathophysiological implications of NETs in intestinal epithelial barrier impairment under hyperglycemic conditions. Most importantly, the findings here demonstrate for the first time that baicalin directly inhibits NETs formation stimulated by high glucose and/or LPS, which may in part account for its pharmacological effects at protecting against hyperglycemia-linked intestinal epithelial barrier impairment.
Copyright © 2025 Cai, Yang, Tang, Wang, Cui, Du, Zhang and Chen.
-
IHC
-
Immunology and Microbiology
In PLoS Pathogens on 1 March 2025 by Florêncio, M., Chagas, M. C. B., et al.
The present study investigates implications of a sub-chromosomal deletion in Leishmania infantum strains, the causative agent of American Visceral Leishmaniasis (AVL). Primarily found in New World strains, the deletion leads to the absence of the ecto-3'-nucleotidase/nuclease enzyme, impacting parasite virulence, pathogenicity, and drug susceptibility. The factors favoring prevalence and the widespread geographic distribution of these deleted mutant parasites (DEL) in the NW (NW) are discussed under the generated data.
We conducted phenotypic assessments of the sub-chromosomal deletion through in vitro assays with axenic parasites and experimental infections in both in vitro and in vivo models of vertebrate and invertebrate hosts using geographically diverse mutant field isolates.
Despite reduced pathogenicity, the DEL strains efficiently infect vertebrate hosts and exhibit relevant differences, including enhanced metacyclogenesis and colonization rates in sand flies, potentially facilitating transmission. This combination may represent a more effective way to maintain and disperse the transmission cycle of DEL strains.
Phenotypic assessments reveal altered parasite fitness, with potential enhanced transmissibility at the population level. Reduced susceptibility of DEL strains to miltefosine, a key drug in VL treatment, further complicates control efforts. The study underscores the importance of typing parasite genomes for surveillance and control, advocating for the sub-chromosomal deletion as a molecular marker in AVL management.
Copyright: © 2025 Florêncio et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
-
FC/FACS
-
Leishmania infantum
-
Immunology and Microbiology
Monocyte control of organismal energy homeostasis
Preprint on BioRxiv : the Preprint Server for Biology on 27 February 2025 by Martins, R., Blankehaus, B., et al.
SUMMARY Multicellular organisms rely on inter-organ communication networks to maintain vital parameters within a dynamic physiological range. Macrophages are central to this homeostatic control system, sensing deviations of those parameters and responding accordingly to support tissue function and organismal homeostasis. Here we demonstrate that dysregulation of iron metabolism in parenchyma cells, imposed by the deletion of ferritin H chain, is sensed by monocyte-derived macrophages. In response, macrophages derived from circulating monocytes support tissue function, energy metabolism and thermoregulation, as demonstrated in bone marrow chimeric and parabiotic mice. This salutary effect is contingent on a transcriptional program, controlled in macrophages by the transcription factor A mitochondria. This transcriptional response acts in a non-cell autonomous manner to support the mitochondria of parenchyma cells, irrespectively of mitochondrial transfer. In conclusion, monocyte-derived macrophages cross-regulate Fe and energy metabolism to support tissue function and organismal homeostasis.
In J Cell Mol Med on 1 March 2020 by Li, H., Tang, C., et al.
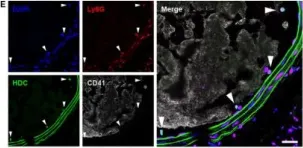
Fig.1.E

-
IHC-IF
-
Mus musculus (House mouse)
Collected and cropped from J Cell Mol Med by CiteAb, provided under a CC-BY license
Image 1 of 5
In J Cell Mol Med on 1 March 2020 by Li, H., Tang, C., et al.
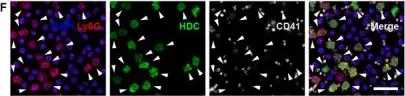
Fig.1.F

-
ICC-IF
-
Mus musculus (House mouse)
Collected and cropped from J Cell Mol Med by CiteAb, provided under a CC-BY license
Image 1 of 5
In Cancer Cell on 13 May 2019 by Segovia, M., Russo, S., et al.
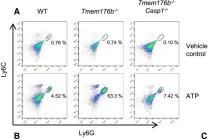
Fig.1.A

-
FC/FACS
-
Mus musculus (House mouse)
Collected and cropped from Cancer Cell by CiteAb, provided under a CC-BY license
Image 1 of 5
In PLoS One on 11 August 2015 by Eichin, D., Laurila, J. P., et al.
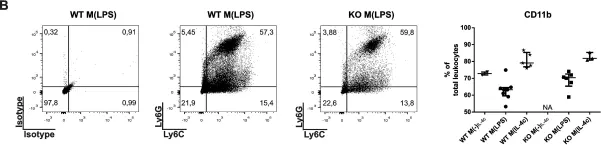
Fig.5.B

-
FC/FACS
-
Mus musculus (House mouse)
Collected and cropped from PLoS One by CiteAb, provided under a CC-BY license
Image 1 of 5
In PLoS One on 11 August 2015 by Eichin, D., Laurila, J. P., et al.
Fig.5.D

-
FC/FACS
-
Mus musculus (House mouse)
Collected and cropped from PLoS One by CiteAb, provided under a CC-BY license
Image 1 of 5