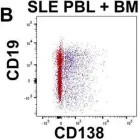
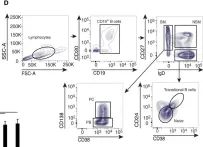
While durable antibody responses from long-lived plasma cell (LLPC) populations are important for protection against pathogens, LLPC may be harmful if they produce antibodies against self-proteins or self-nuclear antigens as occurs in autoimmune diseases such as systemic lupus erythematosus (SLE). Thus, the elimination of autoreactive LLPC may improve the treatment of antibody-driven autoimmune diseases. However, LLPC remain a challenging therapeutic target. Here, we compare the matched bone marrow (BM) and peripheral blood (PBL) plasma cell (PC) compartments of SLE and healthy donors (HD). We show a similar distribution of CD138- and CD138+ PC, including putative LLPC (CD19- CD138+ CD38+), between SLE and HD BM. For both SLE and HD, CD138+ PC are at a higher frequency in BM than PBL. Expression of Ki-67 associates with the PBL compartment where it is found on all PC subsets regardless of CD19 or CD138 expression. Transcriptomic analysis identifies an interferon (IFN) gene signature in transitional B cells in the SLE BM, but surprisingly also in the BM PC derived from SLE. BM PC and B cells phosphorylate STAT1 in response to type I IFN stimulation in vitro, but with decreased fold change compared to those from the PBL. While BM PC bind type I IFN receptor-blocking antibody anifrolumab, it is to a lesser degree than circulating B cells. Anti-nuclear autoantibodies (ANA) are found in the BM supernatant and PBL serum of SLE patients. Both SLE and HD BM-derived PC have increased survival compared to their PBL counterparts when treated with verdinexor. In summary, these findings show evidence of IFN activation in BM PC from SLE.
Copyright © 2025 Alzamareh, Meednu, Nandedkar-Kulkarni, Krenitsky, Barnard, Yasaka, Durrett, Thakar, Rangel-Moreno, Anolik and Barnas.
Product Citations: 26
Interferon activation in bone marrow long-lived plasma cells in systemic lupus erythematosus.
In Frontiers in Immunology on 27 January 2025 by Alzamareh, D. F., Meednu, N., et al.
-
FC/FACS
-
Homo sapiens (Human)
-
Immunology and Microbiology
In Nature Cancer on 1 August 2024 by Ramberger, E., Sapozhnikova, V., et al.
Multiple myeloma (MM) is a plasma cell malignancy of the bone marrow. Despite therapeutic advances, MM remains incurable, and better risk stratification as well as new therapies are therefore highly needed. The proteome of MM has not been systematically assessed before and holds the potential to uncover insight into disease biology and improved prognostication in addition to genetic and transcriptomic studies. Here we provide a comprehensive multiomics analysis including deep tandem mass tag-based quantitative global (phospho)proteomics, RNA sequencing, and nanopore DNA sequencing of 138 primary patient-derived plasma cell malignancies encompassing treatment-naive MM, plasma cell leukemia and the premalignancy monoclonal gammopathy of undetermined significance, as well as healthy controls. We found that the (phospho)proteome of malignant plasma cells are highly deregulated as compared with healthy plasma cells and is both defined by chromosomal alterations as well as posttranscriptional regulation. A prognostic protein signature was identified that is associated with aggressive disease independent of established risk factors in MM. Integration with functional genetics and single-cell RNA sequencing revealed general and genetic subtype-specific deregulated proteins and pathways in plasma cell malignancies that include potential targets for (immuno)therapies. Our study demonstrates the potential of proteogenomics in cancer and provides an easily accessible resource for investigating protein regulation and new therapeutic approaches in MM.
© 2024. The Author(s).
-
Homo sapiens (Human)
In The Journal of Biological Chemistry on 1 August 2024 by Zhu, D., Huang, M. F., et al.
The commitment of stem cells to differentiate into osteoblasts is a highly regulated and complex process that involves the coordination of extrinsic signals and intrinsic transcriptional machinery. While rodent osteoblastic differentiation has been extensively studied, research on human osteogenesis has been limited by cell sources and existing models. Here, we systematically dissect human pluripotent stem cell-derived osteoblasts to identify functional membrane proteins and their downstream transcriptional networks involved in human osteogenesis. Our results reveal an enrichment of type II transmembrane serine protease CORIN in humans but not rodent osteoblasts. Functional analyses demonstrated that CORIN depletion significantly impairs osteogenesis. Genome-wide chromatin immunoprecipitation enrichment and mechanistic studies show that p38 MAPK-mediated CCAAT enhancer binding protein delta (CEBPD) upregulation is required for CORIN-modulated osteogenesis. Contrastingly, the type I transmembrane heparan sulfate proteoglycan SDC1 enriched in mesenchymal stem cells exerts a negative regulatory effect on osteogenesis through a similar mechanism. Chromatin immunoprecipitation-seq, bulk and single-cell transcriptomes, and functional validations indicated that CEBPD plays a critical role in controlling osteogenesis. In summary, our findings uncover previously unrecognized CORIN-mediated CEBPD transcriptomic networks in driving human osteoblast lineage commitment.
Published by Elsevier Inc.
-
Biochemistry and Molecular biology
In Nature Communications on 20 June 2023 by Jiang, D., Huang, H., et al.
BCMA-targeting chimeric antigen receptor (CAR) T cell therapy demonstrates impressive clinical response in multiple myeloma (MM). However, some patients with BCMA-deficient tumours cannot benefit from this therapy, and others can experience BCMA antigen loss leading to relapse, thus necessitating the identification of additional CAR-T targets. Here, we show that FcRH5 is expressed on multiple myeloma cells and can be targeted with CAR-T cells. FcRH5 CAR-T cells elicited antigen-specific activation, cytokine secretion and cytotoxicity against MM cells. Moreover, FcRH5 CAR-T cells exhibited robust tumoricidal efficacy in murine xenograft models, including one deficient in BCMA expression. We also show that different forms of soluble FcRH5 can interfere with the efficacy of FcRH5 CAR-T cells. Lastly, FcRH5/BCMA-bispecific CAR-T cells efficiently recognized MM cells expressing FcRH5 and/or BCMA and displayed improved efficacy, compared with mono-specific CAR-T cells in vivo. These findings suggest that targeting FcRH5 with CAR-T cells may represent a promising therapeutic avenue for MM.
© 2023. The Author(s).
-
FC/FACS
-
Cancer Research
-
Immunology and Microbiology
In Neoplasma on 1 December 2022 by Ramachandran, S. S., Gupta, P., et al.
GRP78 overexpression in myeloma cells has been associated with bortezomib resistance in multiple myeloma (MM). However, serum GRP78 as a maker of bortezomib-based treatment response remains unexplored. The objective of the study was to evaluate serum GRP78 levels in MM patients who underwent a bortezomib-based induction regimen. This cross-sectional study included adult MM patients (n=30) who completed at least four cycles of bortezomib-based induction therapy. Healthy volunteers (n=30) and newly diagnosed MM patients (n=19) were also recruited to identify the disease-associated change in GRP78 levels. Serum GRP78 was estimated by ELISA. Surface and intracellular expression of GRP78 in bone marrow plasma cells was evaluated in ten MM patients by flow cytometry. Among 30 MM patients [median (range): 52 (38-68) years; 20 males] who completed at least four cycles of bortezomib-based induction therapy, 20 were responders and 10 were non-responders. Serum GRP78 levels were not significantly different between responders [median (IQR): 5.2 (3.1, 8.0) μg/ml] and non-responders [median (IQR): 4.3 (0.1, 7.1) μg/ml] (p=0.4). Although non-significant (p=0.3), median serum GRP78 was higher in newly diagnosed patients when compared to healthy volunteers. Bone marrow plasma cells ranged from 0.2 to 57.8% in the analyzed samples. Intracellular GRP78 expression in bone marrow plasma cells was higher (1.6 to 5 times) when compared to surface expression. To conclude, serum GRP78 levels vary widely in different MM patient groups but did not correlate with response to a bortezomib-based induction regimen.
-
FC/FACS
-
Homo sapiens (Human)
In Front Immunol on 27 January 2025 by Alzamareh, D. F., Meednu, N., et al.
Fig.3.B

-
FC/FACS
-
Homo sapiens (Human)
Collected and cropped from Front Immunol by CiteAb, provided under a CC-BY license
Image 1 of 6
In Front Immunol on 27 January 2025 by Alzamareh, D. F., Meednu, N., et al.
Fig.3.A

-
FC/FACS
-
Homo sapiens (Human)
Collected and cropped from Front Immunol by CiteAb, provided under a CC-BY license
Image 1 of 6
In Front Immunol on 26 January 2018 by De, S., Zhang, B., et al.
Fig.1.D

-
FC/FACS
-
Homo sapiens (Human)
Collected and cropped from Front Immunol by CiteAb, provided under a CC-BY license
Image 1 of 6
In Int J Oncol on 1 September 2012 by Kawano, Y., Fujiwara, S., et al.
Fig.1.A

-
FC/FACS
-
Collected and cropped from Int J Oncol by CiteAb, provided under a CC-BY license
Image 1 of 6
In Int J Oncol on 1 September 2012 by Kawano, Y., Fujiwara, S., et al.
Fig.2.D

-
FC/FACS
-
Collected and cropped from Int J Oncol by CiteAb, provided under a CC-BY license
Image 1 of 6
In Int J Oncol on 1 September 2012 by Kawano, Y., Fujiwara, S., et al.
Fig.5.C

-
IHC
-
Collected and cropped from Int J Oncol by CiteAb, provided under a CC-BY license
Image 1 of 6