The role of alveolar macrophages (AMs) in lung carcinogenesis has been extensively studied, yielding significant insights. However, the status of AMs in tumor-bearing lungs remains incompletely characterized. Using orthotopic Lewis Lung Carcinoma (LLC) mouse models, we found that tumors induced an inflammatory extra-tumoral lung microenvironment (ETLME), distinct from the immunosuppressive tumor microenvironment (TME). T cells with an exhaustion phenotype and tumor-associated macrophages (TAMs) mainly accumulated in the TME rather than the ETLME. Surprisingly, AMs were absent from the tumor lesions and remained in the lung tissues, but they displayed a more active dynamic balance between proliferation and death in ETLME. Furthermore, AMs presented an activated phenotype characterized by upregulation of CD11b and downregulation of Siglec-F, elevated expression of inflammatory genes, and enhanced phagocytic and efferocytotic activity. Notably, AMs in ETLME retained their lipid metabolism capacity and responsiveness to external stimuli. More importantly, LLC-experienced AMs display enhanced anti-tumor ability. These findings indicate that AMs maintain their tissue localization and functional integrity within the ETLME.
Copyright © 2025 Ren, Dou, Yue, Ma, Yu, Shang, Wang, Wang, Li and Li.
Product Citations: 83
In Frontiers in Immunology on 8 August 2025 by Ren, M., Dou, J., et al.
-
Cancer Research
-
Immunology and Microbiology
In Nature Communications on 16 April 2025 by Schmidt, A., Fuchs, J., et al.
Lung tissue-resident memory T cells (TRM) are critical for the local control of respiratory tract infections caused by influenza A viruses (IAV). Here we compare TRM populations induced by intranasal adenoviral vector vaccines encoding hemagglutinin and nucleoprotein (NP) with those induced by an H1N1 infection in BALB/c mice. While vaccine-induced TRM express high levels of CD103 and persist longer in the lung parenchyma, short-lived, H1N1-induced TRM have a transcriptome associated with higher cytotoxic potential and distinct transcriptional profile as shown by single-cell RNA sequencing. In both the vaccine and H1N1 groups, NP-specific CD8+ T cells expand during heterologous influenza virus infection and protect the mice from disease. Meanwhile, lung inflammation in response to an infection with unrelated respiratory syncytial virus do not influence the fate of pre-existing TRM. Our preclinical work thus confirms that inflammatory conditions in the tissue shape the phenotypic and functional characteristics of TRM to serve relevant informations for optimizing mucosal vaccines.
© 2025. The Author(s).
-
FC/FACS
-
Mus musculus (House mouse)
-
Immunology and Microbiology
In Nature Communications on 24 January 2025 by Ma, R., Li, Z., et al.
NKp46 is a critical regulator of natural killer (NK) cell immunity, but its function in non-NK innate immune cells remains unclear. Here, we show that NKp46 is indispensable for expressing IL-2 receptor-α (IL-2Rα) by non-NK liver-resident type-1 innate lymphoid cells (ILC1s). Deletion of NKp46 reduces IL-2Rα on ILC1s by downregulating NF-κB signaling, thus impairing ILC1 proliferation and cytotoxicity in vitro and in vivo. The binding of anti-NKp46 antibody to NKp46 triggers the activation of NF-κB, the expression of IL-2Rα, interferon-γ (IFN-γ), tumor necrosis factor (TNF), proliferation, and cytotoxicity. Functionally, NKp46 expressed on mouse ILC1s interacts with tumor cells through cell-cell contact, increasing ILC1 production of IFN-γ and TNF, and enhancing cytotoxicity. In a mouse model of acute myeloid leukemia, deletion of NKp46 impairs the ability of ILC1s to control tumor growth and reduces survival. This can be reversed by injecting NKp46+ ILC1s into NKp46 knock-out mice. Human NKp46+ ILC1s exhibit stronger cytokine production and cytotoxicity than their NKp46- counterparts, suggesting that NKp46 plays a similar role in humans. These findings identify an NKp46-NF-κB-IL-2Rα axis and suggest that activating NKp46 with an anti-NKp46 antibody may provide a potential strategy for anti-tumor innate immunity.
© 2025. The Author(s).
-
Mus musculus (House mouse)
-
Cancer Research
In Nature Communications on 22 October 2024 by Atkin-Smith, G. K., Santavanond, J. P., et al.
Endothelial cells are integral components of all vasculature within complex organisms. As they line the blood vessel wall, endothelial cells are constantly exposed to a variety of molecular factors and shear force that can induce cellular damage and stress. However, how endothelial cells are removed or eliminate unwanted cellular contents, remains unclear. The generation of large extracellular vesicles (EVs) has emerged as a key mechanism for the removal of cellular waste from cells that are dying or stressed. Here, we used intravital microscopy of the bone marrow to directly measure the kinetics of EV formation from endothelial cells in vivo under homoeostatic and malignant conditions. These large EVs are mitochondria-rich, expose the 'eat me' signal phosphatidylserine, and can interact with immune cell populations as a potential clearance mechanism. Elevated levels of circulating EVs correlates with degradation of the bone marrow vasculature caused by acute myeloid leukaemia. Together, our study provides in vivo spatio-temporal characterization of EV formation in the murine vasculature and suggests that circulating, large endothelial cell-derived EVs can provide a snapshot of vascular damage at distal sites.
© 2024. The Author(s).
-
Mus musculus (House mouse)
In Frontiers in Immunology on 17 September 2024 by Zhou, P., Watt, J., et al.
The outbreak of coronavirus disease 19 (COVID-19) has highlighted the demand for vaccines that are safe and effective in inducing systemic and airway mucosal immunity against the aerosol transmission of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). In this study, we developed a novel helper-dependent adenoviral vector-based COVID-19 mucosal vaccine encoding a full-length SARS-CoV-2 spike protein (HD-Ad-FS). Through intranasal immunization (single-dose and prime-boost regimens), we demonstrated that the HD-Ad-FS was immunogenic and elicited potent systemic and airway mucosal protection in BALB/c mice, transgenic ACE2 (hACE2) mice, and hamsters. We detected high titers of neutralizing antibodies (NAbs) in sera and bronchoalveolar lavages (BALs) in the vaccinated animals. High levels of spike-specific secretory IgA (sIgA) and IgG were induced in the airway of the vaccinated animals. The single-dose HD-Ad-FS elicited a strong immune response and protected animals from SARS-CoV-2 infection. In addition, the prime-boost vaccination induced cross-reactive serum NAbs against variants of concern (VOCs; Beta, Delta, and Omicron). After challenge, VOC infectious viral particles were at undetectable or minimal levels in the lower airway. Our findings highlight the potential of airway delivery of HD-Ad-FS as a safe and effective vaccine platform for generating mucosal protection against SARS-CoV-2 and its VOCs.
Copyright © 2024 Zhou, Watt, Mai, Cao, Li, Chen, Duan, Quan, Gingras, Rini, Hu and Liu.
-
FC/FACS
-
Mus musculus (House mouse)
-
COVID-19
-
Immunology and Microbiology
-
Veterinary Research
In Front Immunol on 7 December 2021 by Guo, L., Li, N., et al.
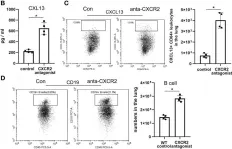
Fig.7.C

-
FC/FACS
-
Mus musculus (House mouse)
Collected and cropped from Front Immunol by CiteAb, provided under a CC-BY license
Image 1 of 5
In Front Immunol on 7 December 2021 by Guo, L., Li, N., et al.
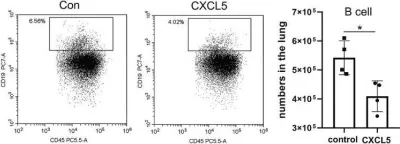
Fig.7.H

-
FC/FACS
-
Mus musculus (House mouse)
Collected and cropped from Front Immunol by CiteAb, provided under a CC-BY license
Image 1 of 5
In Front Immunol on 7 December 2021 by Guo, L., Li, N., et al.
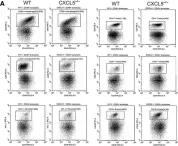
Fig.5.A

-
FC/FACS
-
Mus musculus (House mouse)
Collected and cropped from Front Immunol by CiteAb, provided under a CC-BY license
Image 1 of 5
In Front Immunol on 7 December 2021 by Guo, L., Li, N., et al.
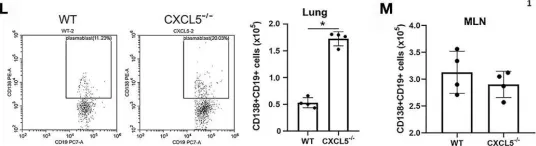
Fig.5.D

-
FC/FACS
-
Mus musculus (House mouse)
Collected and cropped from Front Immunol by CiteAb, provided under a CC-BY license
Image 1 of 5
In Front Immunol on 7 December 2021 by Guo, L., Li, N., et al.
Fig.5.L

-
FC/FACS
-
Mus musculus (House mouse)
Collected and cropped from Front Immunol by CiteAb, provided under a CC-BY license
Image 1 of 5