In humans, Rift Valley fever virus (RVFV) infection typically presents as a self-limiting febrile illness but can cause severe complications. Neurological disease manifestations are particularly concerning as they are associated with increased mortality and long-term morbidity. This study demonstrated that vaccination with live attenuated RVFV was effective in preventing central nervous system (CNS) disease in the CC057/Unc mouse model of late-onset RVF encephalitis. Vaccine candidates (ΔNSs and ΔNSsΔNSm) were safe and immunogenic and elicited both RVFV-specific humoral and cellular immunity. Vaccinated mice survived percutaneous wild-type (WT) RVFV challenge and were protected from CNS disease. Naïve mice that received passive transfer of serum from vaccinated animals 2 days post-WT challenge were protected against late-onset encephalitis. These data demonstrate that humoral immunity is sufficient to protect against RVF encephalitis in CC057/Unc mice and suggest the potential of these vaccine candidates to prevent CNS disease in humans.
© 2025. The Author(s).
Product Citations: 163
In NPJ Vaccines on 2 July 2025 by Mueller Brown, K., Barbeau, D. J., et al.
-
Immunology and Microbiology
In vivo haemopoietic stem cell gene therapy enabled by postnatal trafficking.
In Nature on 1 July 2025 by Milani, M., Fabiano, A., et al.
Lentiviral vector (LV)-mediated ex vivo gene therapy for haematopoietic stem and progenitor cells (HSPCs) has delivered on the promise of a 'one-and-done' treatment for several genetic diseases1. However, ex vivo manipulation and patient conditioning before transplantation are major hurdles that could be overcome by an in vivo approach. Here we demonstrate that in vivo gene delivery to HSPCs after systemic LV administration is enabled by the substantial trafficking of these cells from the liver to the bone marrow in newborn mice. We improved gene-transfer efficiency using a phagocytosis-shielded LV, successfully reaching bona fide HSPCs capable of long-term multilineage output and engraftment after serial transplantation, as confirmed by clonal tracking. HSPC mobilization further increased gene transfer, extending the window of intervention, although permissiveness to LV transduction declined with age. We successfully tested this in vivo strategy in mouse models of adenosine deaminase deficiency, autosomal recessive osteopetrosis and Fanconi anaemia. Interestingly, in vivo gene transfer provided a selective advantage to corrected HSPCs in Fanconi anaemia, leading to near-complete haematopoietic reconstitution and prevention of bone marrow failure. Given that circulating HSPCs in humans are also most abundant shortly after birth, in vivo HSPC gene transfer holds strong translational potential across multiple diseases.
© 2025. The Author(s).
-
Stem Cells and Developmental Biology
In Nature Communications on 1 July 2025 by Perrotta, M., Perrotta, S., et al.
Activated immune cells infiltrate the vasculature during the pathophysiology of hypertension by establishing a vascular-immune interface that contributes to blood pressure dysregulation and organ failure. Many observations indicate a key role of CD8+ T cells in hypertension but mechanisms regulating their activation and interplay with the cardiovascular system are still unknown. In murine model, here we show that a specific member of the phosphoinositide-3-kinases (PI3K) family of lipid kinases, PI3Kγ, is a key intracellular signaling of CD8+ T cells activation and RANTES/CCL5 secretion in hypertension: CCL5-CCR5 signaling is crucial for the establishment of the vascular-immune interface in peripheral organs, lastly contributing to CD8+ tissue infiltration, organ dysfunction and blood pressure elevation. Our studies identify PI3Kγ as a booster of effector CD8+ T cell function, even in the absence of external stimuli. Lastly, an enhanced PI3Kγ signaling mediates the bystander activation of CD8+ T cells and proves effective in transferring the hypertensive phenotype between mice.
© 2025. The Author(s).
-
Cardiovascular biology
-
Immunology and Microbiology
In PLoS ONE on 22 May 2025 by Lee, S. K., Xiong, T., et al.
Homozygous knockout of scavenger receptor class B type I (SR-B1) in mice with atherogenic mutations (such as knockout of the apolipoprotein E or low density lipoprotein receptor genes) results in spontaneous or diet-induced coronary heart disease characterized by atherosclerosis development in the aortic sinus and coronary arteries, platelet accumulation in coronary artery plaques, myocardial fibrosis, and early death. However, the extent of coronary artery atherothrombosis and myocardial fibrosis in mice lacking SR-B1 alone (homozygous SR-B1 knockout mice) has not been examined. Although age is a major risk factor for coronary artery disease, few studies directly examine the effects of age on susceptibility to atherosclerosis or coronary artery atherothrombosis and myocardial fibrosis in mice. Therefore, we set out to examine the effects of age on diet-induced atherosclerosis in female homozygous SR-B1 knockout mice.
SR-B1 knockout mice exhibited little-to-no aortic sinus or coronary artery atherosclerosis at 52 weeks of age, when fed a normal diet. However when fed a high-fat, high-cholesterol, cholate-containing (HFCC) diet for 12 weeks from either 14 weeks of age (26-week-old at analysis) or 40 weeks of age (52-week-old at analysis), they developed similar degrees of atherosclerosis in their aortic sinuses. Interestingly, the older aged SR-B1 knockout mice exhibited increased coronary artery atherosclerosis, increased vascular cell adhesion molecule 1 levels and platelet accumulation in coronary arteries, and increased myocardial fibrosis and plasma levels of cardiac troponin I compared to the younger aged mice. Older-aged HFCC diet-fed SR-B1 knockout mice also exhibited reduced survival to humane endpoint. Moreover, older-aged HFCC diet-fed SR-B1 knockout mice exhibited a greater inflammatory state with increased levels of circulating interleukin-6, tumour necrosis factor alpha, and neutrophils, despite plasma lipid levels being unchanged. Consistent with the increased circulating neutrophils, older-aged HFCC diet-fed SR-B1 knockout mice exhibited increased accumulation of the neutrophil marker myeloperoxidase and increased neutrophil extracellular traps in atherosclerotic plaques in the aortic sinus and increased abundance of atherosclerotic coronary arteries containing neutrophil extracellular traps.
HFCC diet-fed homozygous SR-B1 knockout mice develop occlusive coronary artery atherothrombosis and myocardial fibrosis in an age-dependent manner, and exhibit an increased inflammatory state with older age. Therefore, aged SR-B1 knockout mice may prove to be an attractive mouse model to analyze age-dependent mechanisms associated with coronary artery disease development, which may facilitate the discovery of more effective therapeutics to treat cardiovascular disease.
Copyright: © 2025 Lee et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
-
Cardiovascular biology
β-Arrestin 2 as a Prognostic Indicator and Immunomodulatory Factor in Multiple Myeloma.
In Cells on 26 March 2025 by Mathews, P., Wang, X., et al.
β-arrestin 2 (ARRB2) is involved in the desensitization and trafficking of G protein-coupled receptors (GPCRs) and plays a critical role in cell proliferation, apoptosis, chemotaxis, and immune response modulation. The role of ARRB2 in the pathogenesis of multiple myeloma (MM) has not been elucidated. This study addressed this question by evaluating the expression of ARRB2 in bone marrow (BM) samples from newly diagnosed MM patients and deriving correlations with key clinical outcomes. In light of recent trends towards the use of immune checkpoint inhibitors across malignancies, the effect of ARRB2 in the regulation of the PD-1/PD-L1 axis was also investigated. The expression of ARRB2 was significantly higher in MM patients resistant to proteosome inhibitor (bortezomib) treatment compared to those who responded. Higher ARRB2 expression in the BM of newly diagnosed MM patients was associated with inferior progression-free survival and overall survival. PD-1 expression was downregulated in CD3 T cells isolated from ARRB2 knockout (KO) mice. Furthermore, knockdown of ARRB2 with siRNA reduced PD-1 expression in murine CD3 T cells and PD-L1 expression in murine myeloid-derived suppressor cells. These findings suggest an important role of ARRB2 in MM pathogenesis, potentially mediated via modulation of immune checkpoints in the tumor microenvironment. Our study provides new evidence that ARRB2 may have non-canonical functions independent of GPCRs with relevance to the understanding of MM pathobiology as well as immunotherapy and checkpoint inhibitor escape/resistance more broadly.
-
Cell Biology
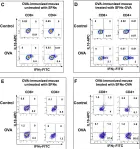
In J Immunother Cancer on 1 January 2023 by Bari, E., Ferrera, F., et al.
Fig.3.C

-
FC/FACS
-
Mus musculus (House mouse)
Collected and cropped from J Immunother Cancer by CiteAb, provided under a CC-BY license
Image 1 of 4
In Sci Rep on 29 September 2017 by Alzubi, J., Pallant, C., et al.
Fig.3.B

-
FC/FACS
-
Mus musculus (House mouse)
Collected and cropped from Sci Rep by CiteAb, provided under a CC-BY license
Image 1 of 4
In Sci Rep on 29 September 2017 by Alzubi, J., Pallant, C., et al.
Fig.4.B

-
FC/FACS
-
Mus musculus (House mouse)
Collected and cropped from Sci Rep by CiteAb, provided under a CC-BY license
Image 1 of 4
In PLoS One on 2 February 2016 by Kicielińska, J., Szczygieł, A., et al.
Fig.6.B

-
FC/FACS
-
Collected and cropped from PLoS One by CiteAb, provided under a CC-BY license
Image 1 of 4