Immunocytokines targeting immune checkpoints have shown great potential in overcoming resistance to immune checkpoint blockade (ICB), making them a major focus of development in recent years. However, severe dose-limiting toxicity hindered their clinical application. Therefore, it is vital to develop versatile strategies to improve safety and elucidate the underlying mechanisms of resistance reversal for advancing immunocytokine therapy.
A general prodrug platform was established to construct interleukin (IL)-15 and IL-12-based immunocytokine prodrugs (P-T, P-Y, and P-Y-IL12). The efficacy of the masking strategy was validated by in vitro activity assays and in vivo safety evaluations. Antitumor efficacy of P-T was assessed in two murine cold tumor models. A comprehensive immune correlate analysis was conducted in the tumor and tumor-draining lymph node (TDLN) to identify key effector cells responsible for overcoming resistance, followed by further confirmation with egression of T cell blockade, surgical excision of TDLN, and adoptive transfer experiments. Finally, the synergistic antitumor effects of P-T with other ICB or HPK1 inhibitors were investigated.
P-T or P-Y using steric hindrance from antibody moiety Fab and Fc shields IL-15 activity in circulation, and reactivates it on cleavage by tumor-specific proteases. The universality of this masking strategy is also applicable to IL-12. Compared with prior constructs, P-T and P-Y exhibit prolonged half-life and tumor retention, facilitating sustained intratumoral immune response. P-T demonstrates reduced systemic toxicity but better control of established tumors over the unmasked counterpart. CD44+ CD8+ T cells in TDLNs are identified as critical mediators of P-T's efficacy: blockade of CD44+ CD8+ T cell trafficking into the tumor microenvironment (TME) markedly diminishes its antitumor effects. On P-T treatment, CD44+ CD8+ T cells exhibit enhanced proliferation in TDLNs and improved antitumor activity. Furthermore, P-T combined with other immunotherapies enhances antitumor effects by increasing CD44+ CD8+ T cells in TDLNs or promoting their infiltration into the TME.
The Fab and Fc-masked prodrug serves as a universal strategy for next-generation immunocytokines design, effectively addressing their dose-limiting toxicity. Additionally, leveraging immunocytokines to mobilize T cells in TDLNs offers a promising therapy option to overcome resistance to ICB and HPK1 inhibitors.
© Author(s) (or their employer(s)) 2025. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ Group.
Product Citations: 124
In Journal for Immunotherapy of Cancer on 31 July 2025 by Song, L., Qi, G., et al.
-
Cancer Research
-
Immunology and Microbiology
In Nature Communications on 1 July 2025 by Perrotta, M., Perrotta, S., et al.
Activated immune cells infiltrate the vasculature during the pathophysiology of hypertension by establishing a vascular-immune interface that contributes to blood pressure dysregulation and organ failure. Many observations indicate a key role of CD8+ T cells in hypertension but mechanisms regulating their activation and interplay with the cardiovascular system are still unknown. In murine model, here we show that a specific member of the phosphoinositide-3-kinases (PI3K) family of lipid kinases, PI3Kγ, is a key intracellular signaling of CD8+ T cells activation and RANTES/CCL5 secretion in hypertension: CCL5-CCR5 signaling is crucial for the establishment of the vascular-immune interface in peripheral organs, lastly contributing to CD8+ tissue infiltration, organ dysfunction and blood pressure elevation. Our studies identify PI3Kγ as a booster of effector CD8+ T cell function, even in the absence of external stimuli. Lastly, an enhanced PI3Kγ signaling mediates the bystander activation of CD8+ T cells and proves effective in transferring the hypertensive phenotype between mice.
© 2025. The Author(s).
-
Cardiovascular biology
-
Immunology and Microbiology
In Cancer Medicine on 1 June 2025 by Li, X., Hua, D., et al.
Breast cancer is the most common malignant tumor among women. Recent studies have found that gut probiotics and their metabolic products play a significant role in activating the immune system, reshaping the tumor microenvironment, and inhibiting cancer progression.
We established a 4T1 tumor-bearing mice model and analyzed the proportions of CD4+ T and CD8+ T cells in the spleen using flow cytometry and immunohistochemistry. The expression levels of TNF-α, IL-6, and IL-10 were measured by enzyme-linked immunosorbent assay. Hematoxylin-eosin staining was used to observe the tumor morphology. Selective protein blotting and quantitative real-time PCR were used to analyze the expression of Bax, Bcl-2, and Caspase-3. Cell proliferation was evaluated using the MTT assay, and apoptosis was detected by flow cytometry.
The results indicated that oral administration of CB and AKK possesses the capability to inhibit the progression of 4T1 breast cancer; however, the combined treatment with both strains (CB-AKK) exhibited significantly superior effects compared to each individual strain. Further mechanistic analysis revealed that the CB-AKK combination could activate the antitumor immunity in mice and reshape the tumor microenvironment. Additionally, it was found that the live bacteria and their metabolites derived from CB-AKK could inhibit cell proliferation and promote tumor apoptosis by activating the Bcl-2/Bax signaling pathway.
This study is the first to demonstrate that orally administered live bacteria CB-AKK can inhibit the progression of 4T1 breast cancer, providing a promising new strategy for the development of innovative biotherapies for breast cancer.
© 2025 The Author(s). Cancer Medicine published by John Wiley & Sons Ltd.
-
Cancer Research
-
Immunology and Microbiology
In Discov Oncol on 10 May 2025 by Ding, C., Li, Z., et al.
Tumor occurrence and growth are highly correlated with the degree of inflammation and immunological activity. Reducing the level of inflammation in tumor-bearing body to relieve immune suppression and enhance anti-tumor immune function has become an important strategy for tumor treatment.
To investigate the effect of albumin-bound paclitaxel combined with Sophora subprostrate polysaccharide (SSP) on inhibiting inflammation, reducing immunosuppression, enhancing anti-tumor immune function and slowing the progression of tumor in tumor-bearing rats, and to provide certain scientific basis for the clinical application of combined drugs in tumor.
The rats were put into three groups at random: normal control, model group, and drug treatment group. After the end of drug intervention, the tumor was taken out and weighed to observe the tumor growth of the rats. Tumor necrosis factor (TNF-α), interleukin (IL) 1β, IL-10, perforin, and granzyme B were found by Western blot in the local tumor tissues of experimental rats. The protein expression levels of Arginase-1 (Arg-1) and Cyclooxygenase 2 (COX-2) were determined. HE staining was used to observe the inflammatory infiltration of the tumor. Using flow cytometry, the proportions of anti-tumor immune cells-CD8 + T cells, NK cells, and immunosuppressive cells-in local tumor tissues were evaluated. In addition, spleen T cells isolated from normal rats were co-cultured with spleen myeloid derived suppressor cells (MDSC) from tumor-bearing rats in the model group and the combined treatment group. Cell Trace Far Red was used to identify T cell proliferation, flow cytometry was used to determine the level of T cell activation from CD25 expression, and in vivo immunosuppression in tumor-bearing rats was examined.
The combined therapy group experienced a considerable decrease in tumor weight as compared to the model group. TNF-α and IL-1p levels in the vicinity of the tumor tissues reduced following intervention, although IL-10 levels, which are anti-inflammatory cytokines, did not significantly change. The results of the HE staining revealed that the intervention group's tumor had less inflammatory infiltration than the model group did. After intervention, the percentages of CD8 + T cells and NK cells in local tumor tissues increased. Additionally, the intervention group's levels of protein expression for perforin and granzyme B were considerably higher than those of the model group. In the nearby tumor tissues, there were lots of MDSC. Following the intervention, the proportion of MDSC in the local tumor tissues was significantly reduced, and the expansion of MDSC was reduced. Additionally, the intervention group's COX-2 and Arg-1 protein expression levels in the tumor-specific tissues were significantly lower than those of the model group. The outcomes of in vitro co-culture demonstrated that rats in the combination group had higher levels of T cell proliferation and activation than animals in the model group.
Albumin-bound paclitaxel combined with Sophora subprostrate polysaccharide can reduce the local inflammation level, promote the proportion of CDB + T cells and NK cells and cell killing function, reduce the proportion of MDSC and immunosuppressive level, enhance the anti-tumor immune function of tumor-bearing mice, and slow the growth of tumors.
© 2025. The Author(s).
-
Cancer Research
-
Immunology and Microbiology
Preprint on BioRxiv : the Preprint Server for Biology on 16 December 2024 by Thomae, T. L., Tan, S. H., et al.
SL/Kh mice develop a high frequency of retrovirally-induced pre-B lymphomas at 3-6 months of age. They also exhibit an abnormal transient expansion of pre-B cells in the bone marrow, although the relevance of this expansion for lymphomagenesis has remained unclear. Here, we use a dual approach that combines pathology with flow cytometry to more fully characterize the nature and origin of SL/Kh lymphomas. Unexpectedly, our studies showed that SL/Kh lymphomas arise from a rare population of pro/pre-B cells in the thymus. We also identified a 10-fold reduction in Notch1 expression in SL/Kh thymic T cells that is associated with a block in early T cell development, a reduction in the number of thymic T cells with age, and an expansion of thymic pro/pre-B cells. This phenotype is consistent with previous studies showing that Notch1 signaling is essential for lymphoid progenitors to undergo T cell commitment and for suppressing B cell development in the thymus. We propose that this developmental defect provides a niche for early B cells to accumulate in the thymus, which, when combined with subsequent retroviral insertional mutagenesis, results in the induction of pre-B lymphomas that originate in the thymus. This is also consistent with our analysis of the genes insertionally mutated in SL/Kh lymphomas, which shows that many function in signaling pathways such as JAK/STAT and RAS/MAPK/ERK that are commonly deregulated in B-cell lymphomas. Primary human mediastinal large B-cell lymphoma (MLBCL) is another lymphoma that is derived from thymic B cells, although virtually nothing is known about the cause of this rare disease. Our studies provide new insights into an underappreciated class of B-cell lymphomas and a mouse model for the study of MLBCL.
-
Mus musculus (House mouse)
-
Cancer Research
-
Immunology and Microbiology
In Cells on 2 January 2024 by Miles, M. A., Luong, R., et al.
Fig.4.B

-
FC/FACS
-
Mus musculus (House mouse)
Collected and cropped from Cells by CiteAb, provided under a CC-BY license
Image 1 of 4
In Cells on 2 January 2024 by Miles, M. A., Luong, R., et al.
Fig.2.B

-
FC/FACS
-
Mus musculus (House mouse)
Collected and cropped from Cells by CiteAb, provided under a CC-BY license
Image 1 of 4
In PLoS One on 22 April 2014 by Du Roure, C., Versavel, A., et al.
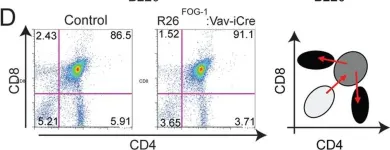
Fig.7.D

-
FC/FACS
-
Collected and cropped from PLoS One by CiteAb, provided under a CC-BY license
Image 1 of 4
In PLoS One on 22 April 2014 by Du Roure, C., Versavel, A., et al.
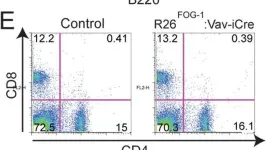
Fig.7.E

-
FC/FACS
-
Collected and cropped from PLoS One by CiteAb, provided under a CC-BY license
Image 1 of 4