The liver is an important metastatic organ that contains many innate immune cells, yet little is known about their role in anti-metastatic defense. We investigated how invariant natural killer T (iNKT) cells influence colorectal cancer-derived liver metastasis using different models in immunocompetent mice. We found that hepatic iNKT cells promote metastasis by creating a supportive niche for disseminated cancer cells. Mechanistically, iNKT cells respond to disseminating cancer cells by producing the fibrogenic cytokines interleukin-4 (IL-4) and IL-13 in a T cell receptor-independent manner. Selective abrogation of IL-4 and IL-13 sensing in hepatic stellate cells prevented their transdifferentiation into extracellular matrix-producing myofibroblasts, which hindered metastatic outgrowth of disseminated cancer cells. This study highlights a novel tumor-promoting axis driven by iNKT cells in the initial stages of metastasis.
© 2025 The Author(s).
Product Citations: 385
In IScience on 16 May 2025 by Nater, M., Brügger, M., et al.
-
Cancer Research
In IScience on 16 May 2025 by Farías, M. A., Cancino, F. A., et al.
Herpes simplex virus type 1 (HSV-1) significantly impairs dendritic cell (DC) function, ultimately eliciting the death of these cells. Here, we sought to assess whether HSV-1 modulates lipid metabolism in mouse DCs as a mechanism of immune evasion. For this, we performed RT-qPCR gene arrays with ingenuity pathway analysis (IPA), RNA sequencing (RNA-seq) and gene set enrichment analysis (GSEA), confocal microscopy, transmission electron microscopy, ultra-high-performance liquid chromatography-quadrupole time-of-flight (UHPLC-QTOF) analysis, pharmacological inhibition of eight lipid-metabolism-related enzymes in HSV-1-infected DCs, co-cultures between virus-specific transgenic CD4+ and CD8+ T cells and HSV-1-infected DCs, and in vivo assays with mice. We found that HSV-1 significantly alters lipid metabolism in DCs and induces lipid droplet (LD) accumulation in these cells. Pharmacological inhibition of two particular lipid metabolism enzymes was found to partially restore DC function. Overall, these results suggest that lipid metabolism plays an important role in the impairment of DC function by HSV-1.
© 2025 The Authors.
-
Biochemistry and Molecular biology
-
Cell Biology
-
Immunology and Microbiology
In Pharmaceutics on 13 May 2025 by Chiu, T. Y., Zho, Y. J., et al.
Background/Objectives: Anti-vascular therapy presents a potential strategy for activating anti-tumor immunity. Disrupted vascular debris provides effective antigens that activate dendritic cells, leading to subsequent immune responses. However, the resulting tumor hypoxia following vascular disruption may contribute to immune suppression, thereby hindering effective immune activation. Ultrasound-stimulated microbubble cavitation can locally disrupt tumor vessels through mechanical effects to achieve physical anti-vascular therapy. Therefore, this study designed oxygen-loaded nanobubbles (ONBs) to combine anti-vascular effects with local oxygen release under ultrasound stimulation. The feasibility of enhancing anti-tumor immune activation by alleviating tumor hypoxia was evaluated. Methods: A murine pancreatic subcutaneous solid tumor model was used to evaluate the efficacy of anti-vascular therapy-associated immunotherapy. Results: After ONB treatment, tumor perfusion was reduced to 52 ± 5%, which resulted in a subsequent 57 ± 11% necrosis and a 29 ± 4% reduction in hypoxia, demonstrating the anti-vascular effect and reoxygenation, respectively. However, subsequent immune responses exhibited no significant activation in intratumoral cytokine expression or splenic immune cell composition. Primary tumors exhibited a 15.7 ± 5.0% increase in necrosis following ONB treatment, but distant tumor growth was not significantly inhibited. Conclusions: These results highlighted a crucial issue regarding the complex correlations between vessel disruption, antigen production, oxygen delivery, hypoxia, and immunity when combining anti-vascular therapy with immunotherapy.
-
Cancer Research
-
Immunology and Microbiology
Local administration of mRNA encoding cytokine cocktail confers potent anti-tumor immunity.
In Frontiers in Immunology on 18 September 2024 by Li, Z., Hu, L., et al.
Immunotherapy using inflammatory cytokines, such as interleukin (IL)-2 and interferon (IFN)-α, has been clinically validated in treating various cancers. However, systemic immunocytokine-based therapies are limited by the short half-life of recombinant proteins and severe dose-limiting toxicities. In this study, we exploited local immunotherapy by intratumoral administration of lipid nanoparticle (LNP)-encapsulated mRNA cocktail encoding cytokines IL-12, IL-7, and IFN-α. The cytokine mRNA cocktail induced tumor regression in multiple syngeneic mouse models and anti-tumor immune memory in one syngeneic mouse model. Additionally, immune checkpoint blockade further enhanced the anti-tumor efficacy of the cytokine mRNAs. Furthermore, human cytokine mRNAs exhibited robust anti-tumor efficacy in humanized mouse tumor models. Mechanistically, cytokine mRNAs induced tumor microenvironment inflammation, characterized by robust T cell infiltration and significant inflammatory cytokine and chemokine production.
Copyright © 2024 Li, Hu, Wang, Liu, Liu, Long, Li, Luo and Peng.
-
FC/FACS
-
Mus musculus (House mouse)
-
Cancer Research
-
Genetics
-
Immunology and Microbiology
In Nature Communications on 25 July 2024 by Park, H., Song, J., et al.
Demyelination due to autoreactive T cells and inflammation in the central nervous system are principal features of multiple sclerosis (MS), a chronic and highly disabling human disease affecting brain and spinal cord. Here, we show that treatment with apelin, a secreted peptide ligand for the G protein-coupled receptor APJ/Aplnr, is protective in experimental autoimmune encephalomyelitis (EAE), an animal model of MS. Apelin reduces immune cell entry into the brain, delays the onset and reduces the severity of EAE. Apelin affects the trafficking of leukocytes through the lung by modulating the expression of cell adhesion molecules that mediate leukocyte recruitment. In addition, apelin induces the internalization and desensitization of its receptor in endothelial cells (ECs). Accordingly, protection against EAE major outcomes of apelin treatment are phenocopied by loss of APJ/Aplnr function, achieved by EC-specific gene inactivation in mice or knockdown experiments in cultured primary endothelial cells. Our findings highlight the importance of the lung-brain axis in neuroinflammation and indicate that apelin targets the transendothelial migration of immune cells into the lung during acute inflammation.
© 2024. The Author(s).
-
FC/FACS
-
Mus musculus (House mouse)
-
Immunology and Microbiology
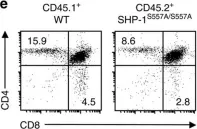
In BMC Cell Biol on 29 September 2018 by Zhu, H., Liu, W., et al.
Fig.3.A

-
FC/FACS
-
Mus musculus (House mouse)
Collected and cropped from BMC Cell Biol by CiteAb, provided under a CC-BY license
Image 1 of 10
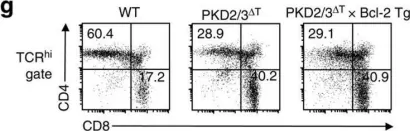
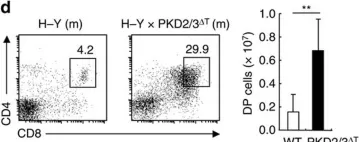
In Nat Commun on 27 September 2016 by Ishikawa, E., Kosako, H., et al.
Fig.3.G

-
FC/FACS
-
Mus musculus (House mouse)
Collected and cropped from Nat Commun by CiteAb, provided under a CC-BY license
Image 1 of 10
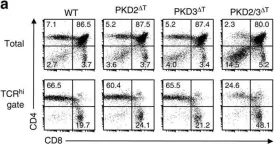
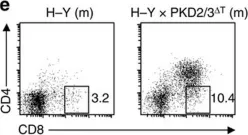
In Nat Commun on 27 September 2016 by Ishikawa, E., Kosako, H., et al.
Fig.3.A

-
FC/FACS
-
Mus musculus (House mouse)
Collected and cropped from Nat Commun by CiteAb, provided under a CC-BY license
Image 1 of 10
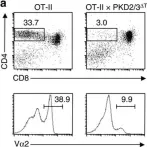
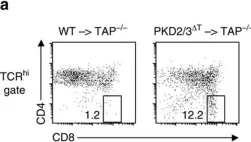
In Nat Commun on 27 September 2016 by Ishikawa, E., Kosako, H., et al.
Fig.4.A

-
FC/FACS
-
Mus musculus (House mouse)
Collected and cropped from Nat Commun by CiteAb, provided under a CC-BY license
Image 1 of 10
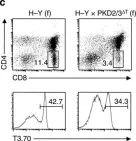
In Nat Commun on 27 September 2016 by Ishikawa, E., Kosako, H., et al.
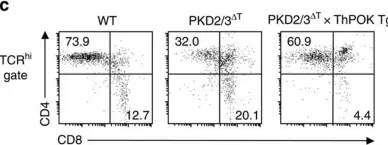
Fig.4.C

-
FC/FACS
-
Mus musculus (House mouse)
Collected and cropped from Nat Commun by CiteAb, provided under a CC-BY license
Image 1 of 10
In Nat Commun on 27 September 2016 by Ishikawa, E., Kosako, H., et al.
Fig.4.D

-
FC/FACS
-
Mus musculus (House mouse)
Collected and cropped from Nat Commun by CiteAb, provided under a CC-BY license
Image 1 of 10
In Nat Commun on 27 September 2016 by Ishikawa, E., Kosako, H., et al.
Fig.4.E

-
FC/FACS
-
Mus musculus (House mouse)
Collected and cropped from Nat Commun by CiteAb, provided under a CC-BY license
Image 1 of 10
In Nat Commun on 27 September 2016 by Ishikawa, E., Kosako, H., et al.
Fig.6.A

-
FC/FACS
-
Mus musculus (House mouse)
Collected and cropped from Nat Commun by CiteAb, provided under a CC-BY license
Image 1 of 10
In Nat Commun on 27 September 2016 by Ishikawa, E., Kosako, H., et al.
Fig.6.C

-
FC/FACS
-
Mus musculus (House mouse)
Collected and cropped from Nat Commun by CiteAb, provided under a CC-BY license
Image 1 of 10
In Nat Commun on 27 September 2016 by Ishikawa, E., Kosako, H., et al.
Fig.10.E

-
FC/FACS
-
Mus musculus (House mouse)
Collected and cropped from Nat Commun by CiteAb, provided under a CC-BY license
Image 1 of 10