Osteosarcoma (OS) with pulmonary metastasis remains challenging due to limited treatment options and the immunosuppressive nature of the tumor microenvironment (TME). Bacteria-mediated cancer therapy has emerged as a promising strategy for solid tumors but often suffers from limited efficacy due to the immunosuppressive TME, which restricts the intensity and durability of the antitumor immune response. To overcome these challenges, we engineered a novel Salmonella strain, VNP20009-CCL2-CXCL9 (VNP-C-C), leveraging the intrinsic tumor tropism of Salmonella typhimurium VNP20009 (VNP) and improving immune modulation through the recruitment of effector immune cells into the TME by the chemokines CCL2 and CXCL9.
VNP-C-C was genetically engineered through electroporation of Plac-CCL2-CXCL9 plasmid and validated in vitro. Its antitumor efficacy, immune regulation capacity and immunomodulatory mechanisms were evaluated in vitro by using OS cell lines and immune cells (dendritic cells (DCs) and macrophages (Mφs)) and in vivo by using both immunocompromised and immunocompetent mouse models of OS lung metastasis.
VNP-C-C effectively accumulated within tumors, triggering immunogenic cell death and subsequently activating the cyclic GMP-AMP synthase (cGAS)/stimulator of interferon genes (STING) pathway, thereby robustly promoting type I interferon secretion. The chemokines CCL2 and CXCL9 amplified the immune response by recruiting DCs, Mφs, and T cells to the TME. This orchestrated immune modulation reprogrammed tumor-associated macrophages to an antitumor phenotype, induced DCs maturation, significantly increased T-cell infiltration and activation within tumors, and promoted systemic T-cell memory formation in peripheral lymphoid organs. These effects collectively inhibited OS lung metastasis progression and provided survival benefits in mouse models.
The engineered bacterial strain VNP-C-C effectively converts the OS lung metastatic TME into a pro-inflammatory milieu, thereby stimulating robust innate and adaptive immune responses. This offers a highly promising therapeutic avenue for OS lung metastasis with considerable translational potential in cancer immunotherapy.
© Author(s) (or their employer(s)) 2025. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ Group.
Product Citations: 314
In Journal for Immunotherapy of Cancer on 1 July 2025 by Liu, R., Liu, Q., et al.
-
FC/FACS
-
Mus musculus (House mouse)
-
Cancer Research
-
Immunology and Microbiology
In CNS Neuroscience Therapeutics on 1 May 2025 by Dai, X., Zhang, X., et al.
Following ischemic stroke, peripheral immune cell infiltration is characterized by myeloid cell predominance in the acute phase and lymphoid cell infiltration in the subacute to chronic phases. Endothelial cells, as a critical interface between the peripheral circulation and the brain, upregulate adhesion molecules to facilitate immune cell infiltration. However, it remains unclear whether endothelial cells exhibit functional differences at different stages after ischemic stroke and how these differences affect immune cell infiltration.
We performed single-cell RNA sequencing on peripheral immune and endothelial cells from Sham and middle cerebral artery occlusion (MCAO) mice at 3 and 14 days post-MCAO. Subsequent analysis of the sequencing data, combined with flow cytometry and immunofluorescence staining, was used to investigate the relationship between endothelial cell changes at different stages of stroke and immune cell infiltration.
We observed that the infiltration capacity of peripheral immune cells did not significantly increase at different stages after MCAO. However, endothelial cells underwent significant changes. By Day 3 post-MCAO, there was an increased proportion of venous endothelial cells with enhanced angiogenesis and adhesion functions. In this acute phase, newly formed venous endothelial cells with high expression of the adhesion molecule ICAM-1 were observed, promoting the infiltration of myeloid cells and NKT cells. From the acute to chronic phases, endothelial angiogenesis gradually decreased, accompanied by a marked increase in antigen presentation function. At 14 days post-MCAO, an increased proportion of VCAM-1-expressing venous endothelial cells was observed, potentially facilitating the infiltration of T cells and a subset of neutrophils. Furthermore, we discovered that the differential changes in venous endothelial cells at different stages after MCAO may be driven by distinct differentiation and proliferation patterns regulated by different signaling pathways.
Our study highlights that the differential expression of adhesion molecules and functional changes in endothelial cells at distinct stages after ischemic stroke may regulate the infiltration patterns of peripheral immune cells.
© 2025 The Author(s). CNS Neuroscience & Therapeutics published by John Wiley & Sons Ltd.
-
Cardiovascular biology
-
Immunology and Microbiology
In Nature Communications on 31 March 2025 by Zhang, X., Li, H., et al.
Ischemic stroke recovery involves dynamic interactions between the central nervous system and infiltrating immune cells. Peripheral immune cells compete with resident microglia for spatial niches in the brain, but how modulating this balance affects recovery remains unclear. Here, we use PLX5622 to create spatial niches for peripheral immune cells, altering the competition between infiltrating immune cells and resident microglia in male mice following transient middle cerebral artery occlusion (tMCAO). We find that early-phase microglia attenuation promotes long-term functional recovery. This intervention amplifies a subset of monocyte-derived macrophages (RAMf) with reparative properties, characterized by high expression of GPNMB and CD63, enhanced lipid metabolism, and pro-angiogenic activity. Transplantation of RAMf into stroke-affected mice improves white matter integrity and vascular repair. We identify Mafb as the transcription factor regulating the reparative phenotype of RAMf. These findings highlight strategies to optimize immune cell dynamics for post-stroke rehabilitation.
© 2025. The Author(s).
-
FC/FACS
-
Mus musculus (House mouse)
-
Cardiovascular biology
-
Neuroscience
In Proceedings of the National Academy of Sciences of the United States of America on 25 March 2025 by Dos S P Andrade, A. C., Lacasse, E., et al.
Platelets, known for maintaining blood balance, also participate in antimicrobial defense. Upon severeacute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection, platelets become hyperactivated, releasing molecules such as cytokines, granule contents, and bioactive lipids. The key effector biolipids produced by platelets include 12-hydroxyeicosatetraenoic acid (12-HETE) and 12-hydroxyeicosatrienoic acid (12-HETrE), produced by 12-lipoxygenase (12-LOX), and prostaglandins and thromboxane, produced by cyclooxygenase-1. While prostaglandin E2 and thromboxane B2 were previously associated with lung inflammation in severe COVID-19, the role of platelet 12-LOX in SARS-CoV-2 infection remains unclear. Using mice deficient for platelets' 12-LOX, we report that SARS-CoV-2 infection resulted in higher lung inflammation characterized by histopathological tissue analysis, increased leukocyte infiltrates, and cytokine production relative to wild-type mice. In addition, distinct platelet and lung transcriptomic changes, including alterations in NOD-like receptor (NLR) family pyrin domain-containing 1 (NLRP1) inflammasome-related gene expression, were observed. Mass spectrometry lipidomic analysis in 12-LOX-deficient-infected mice revealed significant changes in bioactive lipid content, including reduced levels of 12-HETrE that inversely correlated with disease severity. Finally, platelet 12-LOX deficiency was associated with increased morbidity and lower survival rates relative to wild type (WT) mice. Overall, this study highlights the complex interplay between 12-LOX-related lipid metabolism and inflammatory responses during SARS-CoV-2 infection. The findings provide valuable insights into potential therapeutic targets aimed at mitigating severe outcomes, emphasizing the pivotal role of platelet enzymes in the host response to viral infections.
-
COVID-19
-
Immunology and Microbiology
Preprint on BioRxiv : the Preprint Server for Biology on 22 January 2025 by Fusilier, Z., Simon, F., et al.
During tumorigenesis, the extracellular matrix (ECM), which constitutes the structural scaffold of tissues, is profoundly remodeled. While the impact of such remodeling on tumor growth and invasion has been extensively investigated, much less is known on the consequences of ECM remodeling on tumor infiltration by immune cells. By combining tissue imaging and machine-learning, we here show that the localization of T lymphocytes and neutrophils, which orchestrate antitumor immune responses, can be predicted by defined topographical features of fibrillar collagen networks. We further show that these collagen topographies result from the activation of a fibrotic pathway controlled by the transcription factor Tcf4 upon depletion of tumor-associated macrophages at late tumor stages. This pathway promotes the deposition of collagen 3 by both tumor and stromal cells, resulting in intermingled collagen networks that favor intra-tumoral T cell and neutrophil localization. Importantly, analysis of human colorectal cancer public bulk RNAseq databases showed a strong correlation between Tcf4 and collagen 3 , as well as between the expression of these genes and tumor infiltration by T lymphocytes and neutrophils, attesting the clinical relevance of our findings. This study highlights the key structural role of macrophages on the tumor extracellular matrix and identifies collagen network topographies as a major regulator of tumor infiltration by immune cells.
-
Mus musculus (House mouse)
-
Cancer Research
-
Immunology and Microbiology
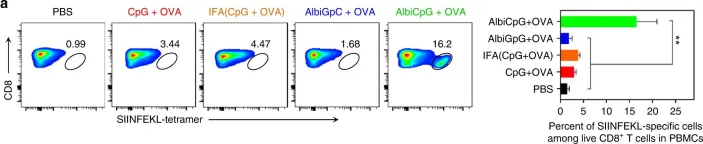
In Nat Commun on 5 December 2017 by Zhu, G., Lynn, G. M., et al.
Fig.4.A

-
FC/FACS
-
Mus musculus (House mouse)
Collected and cropped from Nat Commun by CiteAb, provided under a CC-BY license
Image 1 of 1