Sexual dimorphism in immune responses is well documented, but the underlying mechanisms remain incompletely understood. Here, we identified a subset of corticotropin-releasing hormone (CRH) neurons that express androgen receptors (ARs) as key mediators of sex differences in restraint-induced immunosuppression. Mechanistically, androgens directly activate AR-positive CRH neurons, enhancing the hypothalamic-pituitary-adrenal axis activation. This results in elevated corticosterone levels in response to restraint stress, leading to increased immune cell apoptosis and immune organ atrophy in male mice. Conditional knockout of ARs in CRH neurons eliminated this sexual dimorphism, highlighting ARs in CRH neurons as pivotal regulators of sex-specific immune responses to stress.
Product Citations: 350
In Proceedings of the National Academy of Sciences of the United States of America on 25 March 2025 by Meng, Y., Li, Y., et al.
-
Endocrinology and Physiology
-
Neuroscience
Preprint on BioRxiv : the Preprint Server for Biology on 10 March 2025 by Chen, K., Li, X., et al.
Lipid nanoparticle (LNP)-based mRNA therapeutics, highlighted by the success of SARS-CoV-2 vaccines, face challenges due to inflammation caused by ionizable lipids. These ionizable lipids can activate the immune system, particularly when co-delivered with nucleic acids, leading to undesirable inflammatory responses. We introduce a novel class of anti-inflammatory ionizable lipids functionalized with hydroxychloroquine (HCQ), which suppresses both lipid-induced and nucleic acid-induced immune activation. These HCQ-functionalized LNPs (HL LNPs) exhibit reduced proinflammatory responses while maintaining efficient mRNA delivery. Structural and physicochemical analyses revealed that HCQ-functionalization results in a distinct particle structure with significantly improved stability. The efficacy of HL LNPs was demonstrated across various therapeutic contexts, including a prophylactic vaccination model against varicella-zoster virus (VZV) and CRISPR-Cas9 gene editing targeting PCSK9. Notably, HL LNPs showed robust mRNA expression after repeated administration, addressing concerns of inflammation and ensuring sustained therapeutic effects. These findings highlight the potential of HCQ-functionalized LNPs in expanding the safe use of mRNA therapeutics, particularly for applications requiring repeated dosing and in scenarios where inflammation-induced side effects must be minimized.
-
Genetics
-
Immunology and Microbiology
The UBA1-STUB1 Axis Mediates Cancer Immune Escape and Resistance to Checkpoint Blockade.
In Cancer Discovery on 7 February 2025 by Bao, Y., Cruz, G., et al.
Our study reveals UBA1 as a predictive biomarker for clinical outcomes in ICB cohorts, mediating cancer immune evasion and ICB resistance. We further highlight JAK1 stabilization as a key mechanism of UBA1 inhibition and nominate the UBA1-STUB1 axis as an immuno-oncology therapeutic target to improve the efficacy of ICB.
©2024 The Authors; Published by the American Association for Cancer Research.
-
Cancer Research
-
Immunology and Microbiology
Donor MHC-specific thymus vaccination allows for immunocompatible allotransplantation.
In Cell Research on 1 February 2025 by Liu, Y., Feng, H., et al.
Organ transplantation is the last-resort option to treat organ failure. However, less than 10% of patients benefit from this only option due to lack of major histocompatibility complex (MHC)-matched donor organs and 25%-80% of donated organs could not find MHC-matched recipients. T cell allorecognition is the principal mechanism for allogeneic graft rejection. We herein present a "donor MHC-specific thymus vaccination" (DMTV) strategy to induce T cell tolerance to both autologous and allogeneic donor MHC. Allogeneic MHC molecules were expressed in the recipient thymus through adeno-associated virus-mediated delivery, which led to stable expression of allogeneic MHC together with the autologous MHC in the engineered thymus. During local T cell education, those T cells recognizing either autologous MHC or allogeneic MHC were equally depleted. We constructed C57BL/6-MHC and BALB/c-MHC dual immunocompatible mice via thymus vaccination of C57BL/6-MHC into the BALB/c thymus and observed long-term graft tolerance after transplantation of C57BL/6 skin and C57BL/6 mouse embryonic stem cells into the vaccinated BALB/c mice. We also validated our DMTV strategy in a bone marrow, liver, thymus (BLT)-humanized mouse model for immunocompatible allotransplantation of human embryonic stem cells. Our study suggests that the DMTV strategy is a potent avenue to introduce a donor compatible immune system in recipients, which overcomes the clinical dilemma of the extreme shortage of MHC-matched donor organs for treating patients with end-stage organ failure.
© 2024. The Author(s).
-
Mus musculus (House mouse)
-
Cell Biology
-
Immunology and Microbiology
In Cell Reports Medicine on 21 January 2025 by Wang, H., Zhai, M., et al.
In our previously reported phase 2 and phase 3 studies, the combination of short-course radiotherapy and neoadjuvant immunochemotherapy (SIC) is established as effective cancer therapies for locally advanced rectal cancer (LARC). Here, we apply multi-omic analyses to paired pre- and post-treatment LARC specimens undergoing SIC. The peripheral blood-derived TREM1+ mono-macrophage subsets that display a pro-inflammatory phenotype are identified and correlate with complete response to SIC. Mechanically, ionizing radiation (IR) induces peripheral TREM1+ mono-macrophage expansion in tumors. Following IR, the loss of TREM1 in mono-macrophages undermines antitumor immunity by altering mono-macrophage differentiation and inhibiting CD8+ T cell infiltration and activation. The TREM1+ mono-macrophage response may rely on activation of key inflammatory pathways, including nuclear factor κB (NF-κB) signaling and Toll-like receptor pathway. Pharmacological inhibition of TREM1 signaling abolishes IR-induced immunoactivation and reduces combined IR and/or anti-PD-1 treatment. Thus, we establish a crucial role of a mono-macrophage state in mediating effective cancer therapy.
Copyright © 2024 The Authors. Published by Elsevier Inc. All rights reserved.
-
Cancer Research
-
Cardiovascular biology
In PLoS Pathog on 1 August 2022 by Kim, K. H., Li, Z., et al.
Fig.8.D

-
FC/FACS
-
Collected and cropped from PLoS Pathog by CiteAb, provided under a CC-BY license
Image 1 of 6
In Sci Rep on 13 September 2019 by Jia, S., Li, W., et al.
Fig.6.A

-
FC/FACS
-
Collected and cropped from Sci Rep by CiteAb, provided under a CC-BY license
Image 1 of 6
In Nat Commun on 1 February 2019 by Yasuda, K., Kitagawa, Y., et al.
Fig.1.C

-
FC/FACS
-
Mus musculus (House mouse)
Collected and cropped from Nat Commun by CiteAb, provided under a CC-BY license
Image 1 of 6
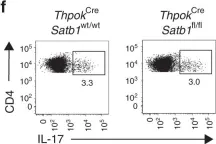
In Nat Commun on 1 February 2019 by Yasuda, K., Kitagawa, Y., et al.
Fig.1.F

-
FC/FACS
-
Mus musculus (House mouse)
Collected and cropped from Nat Commun by CiteAb, provided under a CC-BY license
Image 1 of 6
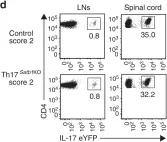
In Nat Commun on 1 February 2019 by Yasuda, K., Kitagawa, Y., et al.
Fig.2.A

-
FC/FACS
-
Mus musculus (House mouse)
Collected and cropped from Nat Commun by CiteAb, provided under a CC-BY license
Image 1 of 6
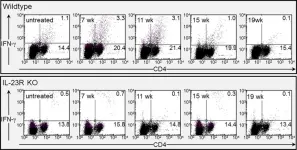
In PLoS One on 18 April 2018 by Caughron, B., Yang, Y., et al.
Fig.8.A

-
FC/FACS
-
Mus musculus (House mouse)
Collected and cropped from PLoS One by CiteAb, provided under a CC-BY license
Image 1 of 6