Cytotoxic lymphocytes are crucial to our immune system, primarily eliminating virus-infected or cancerous cells via perforin/granzyme killing. Perforin forms transmembrane pores in the plasma membrane, allowing granzymes to enter the target cell cytosol and trigger apoptosis. The prowess of cytotoxic lymphocytes to efficiently eradicate target cells has been widely harnessed in immunotherapies against haematological cancers. Despite efforts to achieve a similar outcome against solid tumours, the immunosuppressive and acidic tumour microenvironment poses a persistent obstacle. Using different types of effector cells, including therapeutically relevant anti-CD19 CAR T cells, we demonstrate that the acidic pH typically found in solid tumours hinders the efficacy of immune therapies by impeding perforin pore formation within the immunological synapse. A nanometre-scale study of purified recombinant perforin undergoing oligomerization reveals that pore formation is inhibited specifically by preventing the formation of a transmembrane β-barrel. The absence of perforin pore formation directly prevents target cell death. This finding uncovers a novel layer of immune effector inhibition that must be considered in the development of effective immunotherapies for solid tumours.
© 2025. The Author(s).
Product Citations: 909
Acidic pH can attenuate immune killing through inactivation of perforin.
In EMBO Reports on 1 February 2025 by Hodel, A. W., Rudd-Schmidt, J. A., et al.
-
Immunology and Microbiology
CD28 shapes T cell receptor signaling by regulating Lck dynamics and ZAP70 activation.
In Frontiers in Immunology on 8 January 2025 by Raychaudhuri, K., Rangu, R., et al.
T cell activation requires T cell receptor (TCR) engagement by its specific ligand. This interaction initiates a series of proximal events including tyrosine phosphorylation of the CD3 and TCRζ chains, recruitment, and activation of the protein tyrosine kinases Lck and ZAP70, followed by recruitment of adapter and signaling proteins. CD28 co-stimulation is also required to generate a functional immune response. Currently we lack a full understanding of the molecular mechanism of CD28 activation.
We employed TIRF microscopy to establish detailed spatial and kinetic relationships among these molecules in live Jurkat and murine primary T cells. We used anti-TCR (CD3) antibodies to trigger formation of TCR microclusters (MC), which are submicron-sized basic signaling units formed during T cell activation. Using this model, we aimed to delineate how the CD28 co-stimulatory signal alters the kinetics and molecular stoichiometry of TCR proximal signaling events, and how these effects could affect the immune response.
Our results show that CD28 co-stimulation specifically accelerated recruitment of ZAP70 to the TCRζ chain in MCs and increased ZAP70 activation. CD28-mediated acceleration of ZAP70 recruitment was driven by enhanced Lck recruitment to the MCs. A greater spatial separation between active and inactive species of Lck was also observed in the MCs as a consequence of CD28 co-stimulation.
These results suggest that CD28 co- stimulation may lower the TCR activation threshold by enhancing the activated form of Lck in the TCR MCs.
Copyright © 2024 Raychaudhuri, Rangu, Ma, Alvinez, Tran, Pallikkuth, McIntire, Garvey, Yi and Samelson.
-
Mus musculus (House mouse)
-
Immunology and Microbiology
STAT1 regulates immune-mediated intestinal stem cell proliferation and epithelial regeneration.
In Nature Communications on 2 January 2025 by Takashima, S., Pe'er, D., et al.
The role of the immune system in regulating tissue stem cells remains poorly understood, as does the relationship between immune-mediated tissue damage and regeneration. Graft vs. host disease (GVHD) occurring after allogeneic bone marrow transplantation (allo-BMT) involves immune-mediated damage to the intestinal epithelium and its stem cell compartment. To assess impacts of T-cell-driven injury on distinct epithelial constituents, we have performed single cell RNA sequencing on intestinal crypts following experimental BMT. Intestinal stem cells (ISCs) from GVHD mice have exhibited global transcriptomic changes associated with a substantial Interferon-γ response and upregulation of STAT1. To determine its role in crypt function, STAT1 has been deleted within murine intestinal epithelium. Following allo-BMT, STAT1 deficiency has resulted in reduced epithelial proliferation and impaired ISC recovery. Similarly, epithelial Interferon-γ receptor deletion has also attenuated proliferation and ISC recovery post-transplant. Investigating the mechanistic basis underlying this epithelial response, ISC STAT1 expression in GVHD has been found to correlate with upregulation of ISC c-Myc. Furthermore, activated T cells have stimulated Interferon-γ-dependent epithelial regeneration in co-cultured organoids, and Interferon-γ has directly induced STAT1-dependent c-Myc expression and ISC proliferation. These findings illustrate immunologic regulation of a core tissue stem cell program after damage and support a role for Interferon-γ as a direct contributor to epithelial regeneration.
© 2024. The Author(s).
-
Tissue/organ culture
-
Mus musculus (House mouse)
-
Immunology and Microbiology
-
Stem Cells and Developmental Biology
Th17 cells require the DNA repair sensor XPC to control oxidative DNA damage
Preprint on BioRxiv : the Preprint Server for Biology on 19 September 2024 by Leite, J. A., Notaberardino Bos, N., et al.
Th17 cells are critical for mucosal immunity, producing IL-17A, IL-17F, and IL-22, but dysregulated Th17 responses are implicated in autoimmune diseases. Despite their susceptibility to oxidative stress in certain conditions, Th17 cells exhibit reduced oxidative DNA damage and cell death compared to other T helper subsets. However, the mechanisms that protect Th17 cells from oxidative stress are poorly understood. Here, we identify Xeroderma Pigmentosum Complementation Group C (XPC) as a key regulator of DNA repair and genomic stability in Th17 cells. In XPC-deficient mice, we demonstrate that the absence of XPC impairs Th17 differentiation, as evidenced by reduced expression of key differentiation markers, including Rorc and Il17a, along with decreased IL-17A production. This deficiency leads to increased oxidative stress, DNA damage, and a metabolic shift from glycolysis to oxidative phosphorylation. Moreover, the transcription factor BATF directly regulates XPC expression, linking the BATF-XPC axis to the maintenance of Th17 cell function. Importantly, we find that restoring antioxidant capacity with N-Acetylcysteine (NAC) rescues IL-17A production and reduces DNA damage in XPC-deficient Th17 cells. Mechanistically, we find that XPC interacts with OGG1, a DNA glycosylase involved in the repair of oxidative DNA damage, highlighting XPC’s role in maintaining genomic integrity during Th17 cell differentiation. Our findings reveal a previously unrecognized role for XPC in protecting Th17 cells from oxidative stress, ensuring their proper differentiation and function, with potential implications for targeting DNA repair pathways in autoimmune and inflammatory diseases.
-
Mus musculus (House mouse)
-
Genetics
In IScience on 16 August 2024 by Wenzek, C., Siemes, D., et al.
The immune system has emerged as an important target of thyroid hormones (THs); however, the role of TH in T cells has so far remained elusive. In this study, we assessed the effect of TH receptor α (TRα) signaling on activation and function of T cells. Our findings show that lack of canonical TRα action not only increased the frequency of regulatory T cells (Treg) but propelled an activated and migratory Treg phenotype and nuclear factor κB (NF-κB) activation in Treg. Conversely, canonical TRα action reduced activation of the NF-κB pathway previously shown to play a pivotal role in Treg differentiation and function. Taken together, our findings demonstrate that TRα impacts T cell differentiation and phenotype. Given the well-known interaction of inflammation, immune responses, and TH axis in e.g., severe illness, altered TH-TRα signaling may have an important role in regulating T cell responses during disease.
© 2024 The Author(s).
-
Mus musculus (House mouse)
-
Endocrinology and Physiology
-
Immunology and Microbiology
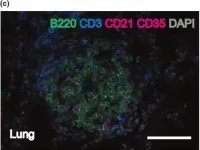
In Immunol Cell Biol on 1 January 2019 by Seillet, C., Arvell, E. H., et al.
Fig.1.C

-
IHC-IF
-
Mus musculus (House mouse)
Collected and cropped from Immunol Cell Biol by CiteAb, provided under a CC-BY license
Image 1 of 3
In Nat Commun on 7 February 2017 by Hu, Y., Lao, L., et al.
Fig.4.C

-
FC/FACS
-
Collected and cropped from Nat Commun by CiteAb, provided under a CC-BY license
Image 1 of 3
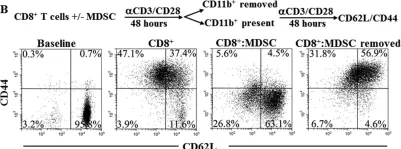
In Oncotarget on 5 April 2016 by Raber, P. L., Sierra, R. A., et al.
Fig.3.B

-
FC/FACS
-
Collected and cropped from Oncotarget by CiteAb, provided under a CC-BY license
Image 1 of 3