Abstract Juvenile myelomonocytic leukemia (JMML), a clonal hematologic malignancy, originates from mutated hematopoietic stem cells (HSCs). The mechanism sustaining the persistence of mutant stem cells, leading to leukemia development, remains elusive. In this study, we conducted comprehensive examination of gene expression profiles, transcriptional factor regulons, and cell compositions/interactions throughout various stages of tumor cell development in Ptpn11 mutation-associated JMML. Our analyses revealed that leukemia-initiating Ptpn11E76K/+ mutant stem cells exhibited de novo activation of the myeloid transcriptional program and aberrant developmental trajectories. These mutant stem cells displayed significantly elevated expression of innate immunity-associated anti-microbial peptides and pro-inflammatory proteins, particularly S100a9 and S100a8. Biological experiments confirmed that S100a9/S100a8 conferred a selective advantage to the leukemia-initiating cells through autocrine effects and facilitated immune evasion by recruiting and promoting immune suppressive myeloid-derived suppressor cells (MDSCs) in the microenvironment. Importantly, pharmacological inhibition of S100a9/S100a8 signaling effectively impeded leukemia development from Ptpn11E76K/+ mutant stem cells. These findings collectively suggest that JMML tumor-initiating cells exploit evolutionarily conserved innate immune and inflammatory mechanisms to establish clonal dominance.
Product Citations: 163
Preprint on Research Square on 2 August 2024 by Zheng, H., Zhao, P., et al.
-
Mus musculus (House mouse)
-
Cancer Research
-
Immunology and Microbiology
-
Stem Cells and Developmental Biology
Immunofilaments Are Well Tolerated after Local or Systemic Administration in Mice.
In ACS Pharmacology & Translational Science on 14 June 2024 by Weiss, L., Classens, R., et al.
The invention of nanosized biomaterials has paved the way for novel therapeutics that can manipulate cells on a nanoscale. Nanosized immunofilaments (IFs) are synthetic filamentous polymers consisting out of polyisocyanopeptides, which have been recently established as a powerful platform to activate specific immune cells in vivo such that they raise an antitumor immune response. However, toxicological effects or immunogenicity toward the IFs have not yet been investigated. In this study, we evaluated potential toxic or immunogenic effects in C57BL/6 mice upon intravenous or subcutaneous injection of nonfunctionalized IFs or immunostimulatory IFs over 30 days. We here present a detailed analysis of the gross pathology, hematological parameters, blood biochemistry, histology, and antibody-response against the IF backbone. Our results demonstrate that IFs do not induce severe acute or chronic toxicity in mice. After 30 days, we only found elevated IgG-titers in intravenously injected but not subcutaneously injected mice. In summary, we demonstrate that IFs can be administered into a living organism without adverse side effects, thereby establishing the safety of IFs as a therapeutic intervention.
© 2024 The Authors. Published by American Chemical Society.
-
Mus musculus (House mouse)
The trogocytosis of neutrophils on initial transplanted tumor in mice.
In IScience on 17 May 2024 by Zhu, M., Wang, S., et al.
The role of neutrophils in tumor initiation stage is rarely reported because of the lack of suitable models. We found that neutrophils recruited in early tumor nodules induced by subcutaneous inoculation of B16 melanoma cells were able to attack tumor cells by trogocytosis. The anti-tumor immunotherapy like peritoneal injection with TLR9 agonist CpG oligodeoxynucleotide combined with transforming growth factor β2 inhibitor TIO3 could increase the trogocytic neutrophils in the nodules, as well as CD8+ T cells, natural killer (NK) cells, and their interferon-γ production. Local use of Cxcl2 small interfering RNA significantly reduced the number of neutrophils and trogocytic neutrophils in tumor nodules, as well as CD8+ T and NK cells, and also enlarged the nodules. These results suggest that neutrophils recruited early to the inoculation site of tumor cells are conducive to the establishment of anti-tumor immune microenvironment. Our findings provide a useful model system for studying the effect of neutrophils on tumors and anti-tumor immunotherapy.
© 2024 The Author(s).
-
FC/FACS
-
Mus musculus (House mouse)
-
Cancer Research
RNA aggregates harness the danger response for potent cancer immunotherapy.
In Cell on 9 May 2024 by Méndez-Gómez, H. R., DeVries, A., et al.
Cancer immunotherapy remains limited by poor antigenicity and a regulatory tumor microenvironment (TME). Here, we create "onion-like" multi-lamellar RNA lipid particle aggregates (LPAs) to substantially enhance the payload packaging and immunogenicity of tumor mRNA antigens. Unlike current mRNA vaccine designs that rely on payload packaging into nanoparticle cores for Toll-like receptor engagement in immune cells, systemically administered RNA-LPAs activate RIG-I in stromal cells, eliciting massive cytokine/chemokine response and dendritic cell/lymphocyte trafficking that provokes cancer immunogenicity and mediates rejection of both early- and late-stage murine tumor models. In client-owned canines with terminal gliomas, RNA-LPAs improved survivorship and reprogrammed the TME, which became "hot" within days of a single infusion. In a first-in-human trial, RNA-LPAs elicited rapid cytokine/chemokine release, immune activation/trafficking, tissue-confirmed pseudoprogression, and glioma-specific immune responses in glioblastoma patients. These data support RNA-LPAs as a new technology that simultaneously reprograms the TME while eliciting rapid and enduring cancer immunotherapy.
Copyright © 2024 Elsevier Inc. All rights reserved.
-
Cancer Research
-
Genetics
-
Immunology and Microbiology
Role of Rad18 in B cell activation and lymphomagenesis.
In Scientific Reports on 25 March 2024 by Kalweit, K., Gölling, V., et al.
Maintenance of genome integrity is instrumental in preventing cancer. In addition to DNA repair pathways that prevent damage to DNA, damage tolerance pathways allow for the survival of cells that encounter DNA damage during replication. The Rad6/18 pathway is instrumental in this process, mediating damage bypass by ubiquitination of proliferating cell nuclear antigen. Previous studies have shown different roles of Rad18 in vivo and in tumorigenesis. Here, we show that B cells induce Rad18 expression upon proliferation induction. We have therefore analysed the role of Rad18 in B cell activation as well as in B cell lymphomagenesis mediated by an Eµ-Myc transgene. We find no activation defects or survival differences between Rad18 WT mice and two different models of Rad18 deficient tumour mice. Also, tumour subtypes do not differ between the mouse models. Accordingly, functions of Rad18 in B cell activation and tumorigenesis may be compensated for by other pathways in B cells.
© 2024. The Author(s).
-
FC/FACS
-
Mus musculus (House mouse)
-
Immunology and Microbiology
In PLoS One on 11 August 2011 by Deswal, S., Schulze, A. K., et al.
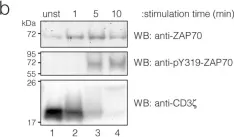
Fig.3.B
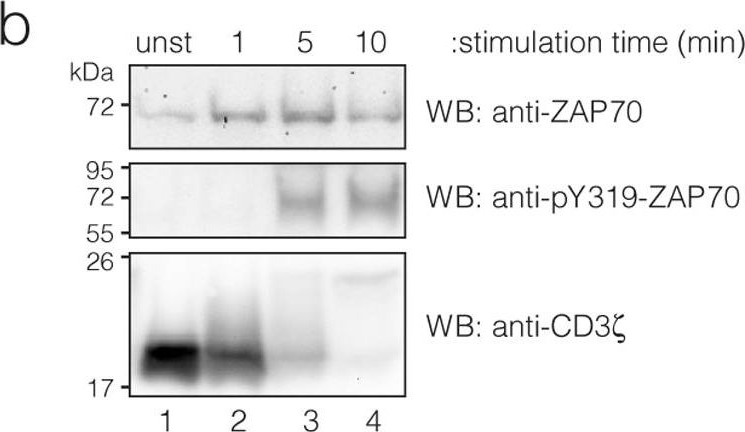
-
WB
-
Mus musculus (House mouse)
Collected and cropped from PLoS One by CiteAb, provided under a CC-BY license
Image 1 of 3
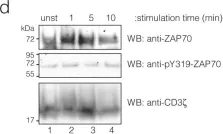
In PLoS One on 11 August 2011 by Deswal, S., Schulze, A. K., et al.
Fig.3.D

-
WB
-
Mus musculus (House mouse)
Collected and cropped from PLoS One by CiteAb, provided under a CC-BY license
Image 1 of 3
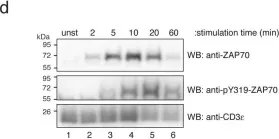
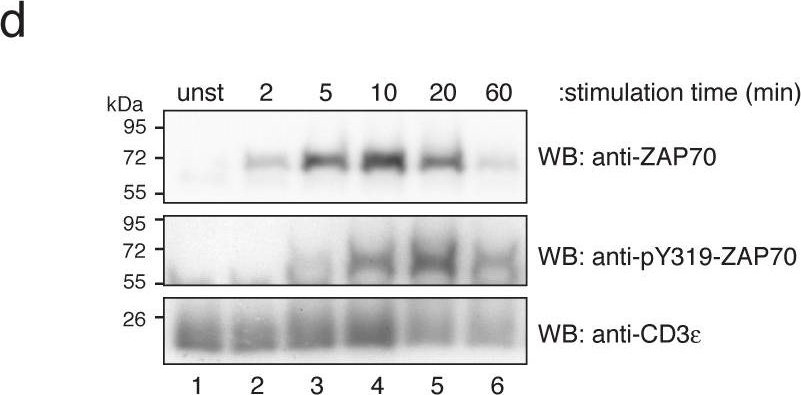
In PLoS One on 11 August 2011 by Deswal, S., Schulze, A. K., et al.
Fig.1.D
-
WB
-
Mus musculus (House mouse)
Collected and cropped from PLoS One by CiteAb, provided under a CC-BY license
Image 1 of 3