NFAT is a family of transcription factors whose activation is inhibited by calcineurin inhibitors (CNIs). In allogeneic hematopoietic stem cell transplantation (allo-HCT), CNIs are employed to prevent and treat graft-versus-host disease (GvHD). Unfortunately, control of cytomegalovirus (CMV), which exacerbates clinical outcomes, is simultaneously lost. Since single NFAT deficiency in T cells ameliorates GvHD in our major mismatch model, we investigated whether protection is maintained during CMV infection. Reassuringly, NFAT-deficient T cells still improved GvHD upon acute CMV infection and after allo-HCT in latently CMV-infected mice, showing reduced proinflammatory and cytotoxic potential. In sharp contrast, CMV-specific NFAT-deficient CD8+ inflated memory T cells expanded more and with higher levels of interferon gamma (IFN-γ) and GzmB expression, effectively controlling CMV. Notably, NFAT-deficient inflated memory T cells could migrate to non-lymphoid tissues and fight CMV. Therefore, CMV infection does not interfere with the protective effect of NFAT inhibition to attenuate GvHD while allowing an anti-CMV response.
© 2025 The Author(s).
Product Citations: 93
In IScience on 21 February 2025 by Hundhausen, N., Majumder, S., et al.
-
Immunology and Microbiology
In Immunity on 12 September 2023 by Brown, H., Komnick, M. R., et al.
Lymph nodes (LNs) are critical sites for shaping tissue-specific adaptive immunity. However, the impact of LN sharing between multiple organs on such tailoring is less understood. Here, we describe the drainage hierarchy of the pancreas, liver, and the upper small intestine (duodenum) into three murine LNs. Migratory dendritic cells (migDCs), key in instructing adaptive immune outcome, exhibited stronger pro-inflammatory signatures when originating from the pancreas or liver than from the duodenum. Qualitatively different migDC mixing in each shared LN influenced pancreatic β-cell-reactive T cells to acquire gut-homing and tolerogenic phenotypes proportional to duodenal co-drainage. However, duodenal viral infections rendered non-intestinal migDCs and β-cell-reactive T cells more pro-inflammatory in all shared LNs, resulting in elevated pancreatic islet lymphocyte infiltration. Our study uncovers immune crosstalk through LN co-drainage as a powerful force regulating pancreatic autoimmunity.
Copyright © 2023 Elsevier Inc. All rights reserved.
-
Immunology and Microbiology
In Science Advances on 18 January 2023 by Charnley, M., Allam, A. H., et al.
A critical stage of T cell development is β-selection; at this stage, the T cell receptor β (TCRβ) chain is generated, and the developing T cell starts to acquire antigenic specificity. Progression through β-selection is assisted by low-affinity interactions between the nascent TCRβ chain and peptide presented on stromal major histocompatibility complex and cues provided by the niche. In this study, we identify a cue within the developing T cell niche that is critical for T cell development. E-cadherin mediates cell-cell interactions and influences cell fate in many developmental systems. In developing T cells, E-cadherin contributed to the formation of an immunological synapse and the alignment of the mitotic spindle with the polarity axis during division, which facilitated subsequent T cell development. Collectively, these data suggest that E-cadherin facilitates interactions with the thymic niche to coordinate the β-selection stage of T cell development.
-
Immunology and Microbiology
Preprint on BioRxiv : the Preprint Server for Biology on 15 June 2022 by Charnley, M., Allam, A. H., et al.
A critical stage of T cell development is β-selection; at this stage the TCRβ chain is generated and the developing T cell starts to acquire antigenic specificity. Progression through β-selection is assisted by a low affinity interaction between the nascent TCRβ chain and peptide presented on stromal MHC and external cues provided by the niche, including Notch and CXCR4. In this study, we reveal the importance of a new cue within the murine developing T cell niche which is critical for T cell development. E-cadherin mediates cell-cell interactions and influences cell fate in many developmental systems. In developing T cells E-cadherin contributed to the formation of an immunological synapse and the alignment of the mitotic spindle with the polarity axis during division, which facilitated subsequent T cell development. Collectively, these data highlight a new aspect of the developing T cell niche and provide insights into the role of E-cadherin in the β-selection stage of T cell development.
-
Mus musculus (House mouse)
-
Immunology and Microbiology
In Cancer Immunology Research on 1 July 2021 by Gkountidi, A. O., Garnier, L., et al.
Several solid malignancies trigger lymphangiogenesis, facilitating metastasis. Tumor-associated lymphatic vessels significantly contribute to the generation of an immunosuppressive tumor microenvironment (TME). Here, we have investigated the ability of tumoral lymphatic endothelial cells (LEC) to function as MHC class II-restricted antigen-presenting cells in the regulation of antitumor immunity. Using murine models of lymphangiogenic tumors engrafted under the skin, we have shown that tumoral LECs upregulate MHC class II and the MHC class II antigen-processing machinery, and that they promote regulatory T-cell (Treg) expansion ex vivo. In mice with LEC-restricted lack of MHC class II expression, tumor growth was severely impaired, whereas tumor-infiltrating effector T cells were increased. Reduction of tumor growth and reinvigoration of tumor-specific T-cell responses both resulted from alterations of the tumor-infiltrating Treg transcriptome and phenotype. Treg-suppressive functions were profoundly altered in tumors lacking MHC class II in LECs. No difference in effector T-cell responses or Treg phenotype and functions was observed in tumor-draining lymph nodes, indicating that MHC class II-restricted antigen presentation by LECs was required locally in the TME to confer potent suppressive functions to Tregs. Altogether, our study suggests that MHC class II-restricted antigen-presenting tumoral LECs function as a local brake, dampening T cell-mediated antitumor immunity and promoting intratumoral Treg-suppressive functions.©2021 American Association for Cancer Research.
-
FC/FACS
-
Mus musculus (House mouse)
-
Cancer Research
-
Immunology and Microbiology
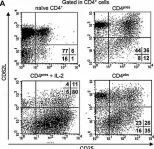
In Eur J Immunol on 1 June 2008 by Oliveira, V., Sawitzki, B., et al.
Fig.4.A

-
FC/FACS
-
Collected and cropped from Eur J Immunol by CiteAb, provided under a CC-BY license
Image 1 of 1