Alzheimer's disease (AD), the leading cause of senile dementia, lacks effective therapies. While microglia are central to AD pathology, key therapeutic targets remain unclear. Here we identify microglial connexin43 (Cx43) hemichannels as a regulator of microglial reactivity in AD, positioning them as a promising therapeutic target. Post-mortem AD patient tissue showed elevated Cx43 levels in periplaque microglia. In the APPswe/PS1dE9 (APP/PS1) mouse model of amyloidosis, we demonstrated that microglial Cx43 hemichannels correlated with microglial malfunction, which in turn exacerbated β-amyloid pathology. Ablation of microglial Cx43 hemichannels by genetic knockout shifts microglia to a neuroprotective phenotype, enhancing the microglia-plaque interaction while suppressing neurotoxicity, thereby mitigating the progression of AD-like pathology. We developed TAT-Cx43@LNPs, a Cx43 hemichannel-targeting peptide delivered by a lipid nanoparticle system, which effectively delayed and rescued β-amyloid-related neuropathology and cognitive impairment in APP/PS1 mice. This study provides evidence for advancing Cx43 hemichannel targeting therapy into clinical trials.
© 2025. The Author(s).
Product Citations: 137
In Nature Communications on 1 July 2025 by Su, Y., Li, H., et al.
-
Neuroscience
Obesity drives depot-specific vascular remodeling in male white adipose tissue.
In Nature Communications on 25 June 2025 by Hasan, S. S., John, D., et al.
Obesity-driven pathological expansion of white adipose tissue (WAT) is a key driver of endothelial dysfunction. However, early vascular alterations associated with over-nutrition also serve to exacerbate WAT dysfunction. Here, we conduct a single-cell transcriptomic analysis of WAT endothelium to delineate endothelial heterogeneity and elucidate vascular alterations and its consequence in a male murine model of obesity. We demarcate depot-specific differences in subcutaneous (sWAT) and visceral WAT (vWAT) endothelium through in sillico analysis and further corroboration of our findings. Moreover, we identify a sWAT-specific fenestrated endothelial cell (EC) subtype, which declines in obese conditions. Utilizing systemic anti-VEGFA blockade and genetic Vegfa manipulation, we demonstrate that VEGFA is necessary for maintaining fenestration in sWAT. Additionally, we detect this fenestrated EC subtype in male human WAT, which undergoes reduction in individuals with obesity. Collectively, this atlas serves as a valuable tool for future studies to decipher the functional significance of different WAT EC subtypes.
© 2025. The Author(s).
Alveolar epithelial and vascular CXCR2 mediates transcytosis of CXCL1 in inflamed lungs.
In Nature Communications on 24 May 2025 by Thomas, K., Rossaint, J., et al.
Pulmonary infections are characterized by neutrophil recruitment into the lung driven by chemokine ligands of CXCR2, which is expressed on neutrophils, but also present in non-hematopoietic lung cells, in which its role remains unclear. We hypothesize that CXCR2 in epithelial and endothelial cells contributes to neutrophil recruitment into the lung by modifying the availability of its cognate chemokines in lung alveoli. Using conditional endothelial and epithelial CXCR2 knockout mice, we demonstrate that selective CXCR2 deletion in either compartment impairs neutrophil recruitment into the lung during bacterial pneumonia and reduces bacterial clearance. We show that CXCR2 ablation in epithelial and endothelial cells compromises respective trans-epithelial and trans-endothelial transcytosis of alveolar CXCL1. Mechanistically, CXCR2-mediated CXCL1 endothelial and epithelial cell transcytosis requires the function of Bruton's tyrosine kinase in these cells. In conclusion, CXCR2 plays an important role in alveolar epithelial and endothelial cells, where it mediates cognate chemokine transcytosis, thus actively supporting their activities in neutrophil recruitment to the infected lungs.
© 2025. The Author(s).
VEGF-E Attenuates Injury After Ischemic Stroke by Promoting Reparative Revascularization.
In European Journal of Neuroscience on 1 April 2025 by Menet, R., Nasrallah, L., et al.
The angiogenic response after stroke correlates with mild injury and an improved recovery. Stimulation of post-stroke angiogenesis using vascular endothelial growth factor (VEGF)-A is associated with an increased risk of vascular destabilization, leading to life-threatening complications. The non-mammalian VEGF-A homolog, VEGF-E, stimulates stable cutaneous vascularization and promotes wound healing. Herein, we posit that VEGF-E represents a potential mediator of reparative revascularization after ischemic stroke. C57BL6/J wildtype mice were subjected to experimental stroke, and VEGF-E or VEGF-A were intranasally delivered during the subacute phase. Our results indicate that VEGF-E improves neurological recovery and increases vascular density without compromising permeability, more efficiently than VEGF-A. We show that VEGF-E-mediated revascularization correlates with normal restoration of brain perfusion, whereas VEGF-A induces cerebral hyperperfusion, indicative of vascular dysfunction. Furthermore, VEGF-E reduces microvascular stalls, increases the density of angiogenic vasculature, and improves the interaction of brain endothelial cell with pericytes, which is critical for vascular stabilization. Using cell-based assays, we demonstrate that stimulation of brain endothelial cells with VEGF-E, but not with VEGF-A, increases the expression of platelet-derived growth factor (PDGF)-D, a potent ligand of PDGFRβ that plays critical roles in regulating the survival and functions of perivascular cells, including pericytes. These effects are associated with activation of extracellular signal-regulated kinase (ERK)1/2 and P38 mitogen-activated protein kinase (MAPK). Finally, we confirm that the secretome of VEGF-E-stimulated brain endothelial cells ameliorates pericyte migration required for vascular recruitment. Our study indicates that VEGF-E promotes a stable and functional revascularization after ischemic stroke, outlining its promises for therapeutic purposes.
© 2025 The Author(s). European Journal of Neuroscience published by Federation of European Neuroscience Societies and John Wiley & Sons Ltd.
-
Cardiovascular biology
-
Neuroscience
Targeted imaging of pulmonary fibrosis by a cyclic peptide LyP-1.
In Scientific Reports on 8 March 2025 by Li, J., Shu, R., et al.
Pulmonary fibrosis (PF) is an interstitial chronic lung disease characterized by interstitial inflammation and extracellular matrix deposition, resulting in progressive lung dysfunction and ultimate respiratory failure. However, lacking of precise and noninvasive tracers for fibrotic lesions limits timely diagnosis and treatment. Here, we identified LyP-1, a cyclic peptide, as a specific and sensitive tracer for PF detection using PET/CT imaging. FITC-LyP-1 selectively recognized fibrotic regions in bleomycin-induced PF mice, indicating its targeting capability. The colocalization of FITC-LyP-1 with extracellular collagen I within the fibrotic lesions validated its specificity, and further analysis revealed several potential target molecules. In the in vivo application studies, radiolabeled [68Ga]Ga-LyP-1 showed significantly increased lung uptake in PF mice, specifically enriching fibrotic regions on PET/CT imaging. Notably, compared to CT imaging that showed increased mean lung density throughout the phases after bleomycin-administration, lung uptake of [68Ga]Ga-LyP-1 was only increased in the later phase, indicating that LyP-1 recognizes the fibrous changes rather than the inflammatory cells in vivo. These results suggest that the new radiotracer [68Ga]Ga-LyP-1 specifically detects the extracellular matrix in fibrotic lungs. LyP-1 shows promise as a noninvasive tracer for assessing human pulmonary fibrosis, offering potential for improved diagnostic accuracy and timely intervention.
© 2024. The Author(s).
-
IHC-P-IF
-
Mus musculus (House mouse)
-
Cardiovascular biology
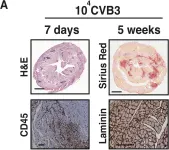
In PLoS One on 2 April 2019 by Deckx, S., Johnson, D. M., et al.
Fig.3.A

-
IHC
-
Mus musculus (House mouse)
Collected and cropped from PLoS One by CiteAb, provided under a CC-BY license
Image 1 of 3
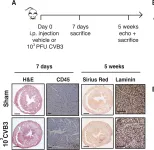
In PLoS One on 2 April 2019 by Deckx, S., Johnson, D. M., et al.
Fig.2.A

-
IHC
-
Mus musculus (House mouse)
Collected and cropped from PLoS One by CiteAb, provided under a CC-BY license
Image 1 of 3
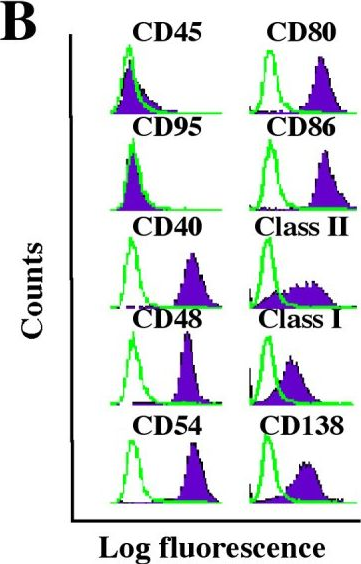
In Mol Cancer on 9 November 2005 by Han, S. S., Shaffer, A. L., et al.
Fig.1.B
-
FC/FACS
-
Mus musculus (House mouse)
Collected and cropped from Mol Cancer by CiteAb, provided under a CC-BY license
Image 1 of 3