We previously showed correction of sickle cell anemia (SCA) in mice utilizing a lentiviral vector (LV) expressing human γ-globin. Herein, we made a G16D mutation in the γ-globin gene to generate the G16D mutation (GbGM) LV to increase fetal hemoglobin formation. We also generated an insulated version of this LV, GbGMI, inserting a 36-bp insulator from the Foamy virus in the long terminal repeats of the LV. Preclinical batches of GbGM and GbGMI LV showed both were highly efficacious in correcting SCA in mice, with sustained gene transfer in primary transplanted SCA mice and high hematopoietic stem cell (HSC) transduction in colony-forming unit-spleen in secondary transplanted mice. CRISPR-mediated targeting of the proviruses into the LMO2 proto-oncogene showed remarkably reduced LMO2 activation by both insulated and uninsulated LV, compared to the SFFV γ-RV vector targeted to the same locus. We therefore used the GbGM LV to perform preclinical human CD34+ gene transfer. We assessed gene transfer and engraftment of human HSCs in two immunocompromised mouse models: persistent stable GbGM-transduced cell engraftment was comparable to that of untransduced cells with no detrimental effects on hematopoiesis up to 20 weeks post transplant. These robust preclinical studies in mouse and human HSCs allowed its translation into a clinical trial.
© 2025 The Authors.
Product Citations: 80
In Molecular Therapy. Methods Clinical Development on 12 June 2025 by Shrestha, A., Pillis, D. M., et al.
Fibroblast hierarchy dynamics during mammary gland morphogenesis and tumorigenesis.
In The EMBO Journal on 1 June 2025 by Pascual, R., Cheng, J., et al.
Fibroblasts form a major component of the stroma in normal mammary tissue and breast tumors. Here, we have applied longitudinal single-cell transcriptome profiling of >45,000 fibroblasts in the mouse mammary gland across five different developmental stages and during oncogenesis. In the normal gland, diverse stromal populations were resolved, including lobular-like fibroblasts, committed preadipocytes and adipogenesis-regulatory, as well as cycling fibroblasts in puberty and pregnancy. These specialized cell types appear to emerge from CD34high mesenchymal progenitor cells, accompanied by elevated Hedgehog signaling. During late tumorigenesis, heterogeneous cancer-associated fibroblasts (CAFs) were identified in mouse models of breast cancer, including a population of CD34- myofibroblastic CAFs (myCAFs) that were transcriptionally and phenotypically similar to senescent CAFs. Moreover, Wnt9a was demonstrated to be a regulator of senescence in CD34- myCAFs. These findings reflect a diverse and hierarchically organized stromal compartment in the normal mammary gland that provides a framework to better understand fibroblasts in normal and cancerous states.
© 2025. The Author(s).
Proinflammatory cytokines sensitise mesenchymal stromal cells to apoptosis.
In Cell Death Discovery on 28 March 2025 by Payne, N. L., Pang, S. H. M., et al.
Mesenchymal stromal cells (MSCs) exert broad therapeutic effects across a range of inflammatory diseases. Their mechanism of action has largely been attributed to paracrine signalling, orchestrated by an array of factors produced by MSCs that are collectively termed the "secretome". Strategies to enhance the release of these soluble factors by pre-exposure to inflammatory cytokines, a concept known as "licensing", is thought to provide a means of enhancing MSC efficacy. Yet, recent evidence shows that intravenously infused MSCs entrapped within the lungs undergo apoptosis, and their subsequent clearance by host phagocytes is essential for their therapeutic efficacy. We therefore sought to clarify the mechanisms governing regulated cell death in MSCs and how exposure to inflammatory cytokines impacts this process. Our results show that MSCs are relatively resistant to cell death induced via the extrinsic pathway of apoptosis, as well as stimuli that induce necroptosis, a form of regulated inflammatory cell death. Instead, efficient killing of MSCs required triggering of the mitochondrial pathway of apoptosis, via inhibition of the pro-survival proteins MCL-1 and BCL-XL. Apoptotic bodies were readily released by MSCs during cell disassembly, a process that was inhibited in vitro and in vivo when the apoptotic effectors BAK and BAX were genetically deleted. Licensing of MSCs by pre-exposure to the inflammatory cytokines TNF and IFN-γ increased the sensitivity of MSCs to intrinsic apoptosis in vitro and accelerated their in vivo clearance by host cells within the lungs after intravenous infusion. Taken together, our study demonstrates that inflammatory "licensing" of MSCs facilitates cell death by increasing their sensitivity to triggers of the intrinsic pathway of apoptosis and accelerating the kinetics of apoptotic cell disassembly.
© 2025. The Author(s).
Surfactant Protein-C Regulates Alveolar Type 2 Epithelial Cell Lineages via the CD74 Receptor.
In Journal of Respiratory Biology and Translational Medicine on 1 December 2024 by Jain, K. G., Liu, Y., et al.
Deficiency of surfactant protein-C (SPC) increases susceptibility to lung infections and injury, and suppressed expression of SPC has been associated with the severity of acute respiratory distress syndrome (ARDS). Alveolar type 2 epithelial cells (AT2) are critical for maintenance and repair of the lung. However, the role of the SPC in the regulation of AT2 cell lineage and the underlying mechanisms are not completely understood.
This study aimed to investigate the mechanisms by which SPC regulates AT2 lineages. Sftpc-/- mice were used to model the SPC deficiency in ARDS patients. We utilized three-dimensional (3D) organoids to compare AT2 lineage characteristics between wild type (WT) and Sftpc-/- mice by analyzing AT2 proliferation, alveolar type 1 cells (AT1) differentiation and CD74 expression, using colony-formation assay, immunofluorescence, flow cytometry, and immunoblots.
The results showed that Sftpc-/- mice demonstrated a reduced AT2 cell population. Influenza A virus subtype H1N1 (H1N1) infected Sftpc-/- mice demonstrated reduced AT2 proliferation and AT1 differentiation. Western blot indicated elevated levels of CD74 protein in AT2 cells of Sftpc-/- mice. Colony-forming efficiency was significantly attenuated in AT2 cells isolated from Sftpc-/- mice compared to the WT controls. Podoplanin (PDPN, a marker of AT1 cells) expression and transient cell count significantly increased in Sftpc-/- organoids. Moreover, siRNA-mediated gene silencing of CD74 in AT2 cells significantly increased AT2 proliferation and AT1 differentiation in Sftpc-/- organoids.
This study suggests that SPC regulates AT2 lineage in vitro and in vivo. The SPC might influence AT2 lineage during the lung epithelium repair by activating signaling mechanism involving CD74 receptor.
In Cell Stem Cell on 3 October 2024 by Xu, L., Tan, C., et al.
While all eukaryotic cells are dependent on mitochondria for function, in a complex tissue, which cell type and which cell behavior are more sensitive to mitochondrial deficiency remain unpredictable. Here, we show that in the mouse airway, compromising mitochondrial function by inactivating mitochondrial protease gene Lonp1 led to reduced progenitor proliferation and differentiation during development, apoptosis of terminally differentiated ciliated cells and their replacement by basal progenitors and goblet cells during homeostasis, and failed airway progenitor migration into damaged alveoli following influenza infection. ATF4 and the integrated stress response (ISR) pathway are elevated and responsible for the airway phenotypes. Such context-dependent sensitivities are predicted by the selective expression of Bok, which is required for ISR activation. Reduced LONP1 expression is found in chronic obstructive pulmonary disease (COPD) airways with squamous metaplasia. These findings illustrate a cellular energy landscape whereby compromised mitochondrial function could favor the emergence of pathological cell types.
Copyright © 2024 Elsevier Inc. All rights reserved.
-
Cell Biology
-
Stem Cells and Developmental Biology
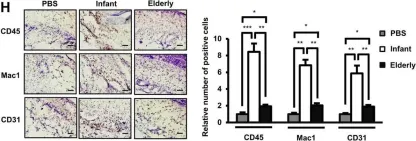
In Sci Rep on 14 October 2020 by Khanh, V. C., Yamashita, T., et al.
Fig.1.H

-
IHC
-
Collected and cropped from Sci Rep by CiteAb, provided under a CC-BY license
Image 1 of 1