Recent studies have revealed a structural role for DNA ligase 4 (Lig4) in the maintenance of a repair complex during non-homologous end joining (NHEJ) of DNA double-strand breaks. In cultured cell lines, catalytically inactive Lig4 can partially alleviate the severe DNA repair phenotypes observed in cells lacking Lig4. To study the structural role of Lig4 in vivo, a mouse strain harboring a point mutation to Lig4's catalytic site was generated. In contrast to the ablation of Lig4, catalytically inactive Lig4 mice are born alive. These mice display marked growth retardation and have clear deficits in lymphocyte development. We considered that the milder phenotype results from inactive Lig4 help to recruit another ligase to the repair complex. We next generated a mouse strain deficient for nuclear Lig3. Nuclear Lig3-deficient mice are moderately smaller and have elevated incidences of cerebral ventricle dilation but otherwise appear normal. Strikingly, in experiments crossing these two strains, mice lacking nuclear Lig3 and expressing inactive Lig4 were not obtained. Timed mating revealed that fetuses harboring both mutations underwent resorption, establishing an embryonic lethal genetic interaction. These data suggest that Lig3 is recruited to NHEJ complexes to facilitate end joining in the presence (but not activity) of Lig4.
© The Author(s) 2024. Published by Oxford University Press on behalf of Nucleic Acids Research.
Product Citations: 168
In Nucleic Acids Research on 11 January 2025 by Medina-Suárez, D., Han, L., et al.
-
Biochemistry and Molecular biology
-
Genetics
CD30 influences germinal center B-cell dynamics and the expansion of IgG1-switched B cells.
In Cellular Molecular Immunology on 1 December 2024 by Wang, Y., Rambold, U., et al.
Initially, identified as a Hodgkin lymphoma marker, CD30 was subsequently detected on a subset of human B cells within and around germinal centers (GCs). While CD30 expression is typically restricted to a few B cells, expansion of CD30-expressing B cells occurs in certain immune disorders and during viral infections. The role of CD30 in B cells remains largely unclear. To address this gap in knowledge, we established a conditional CD30-knockin mouse strain. In these mice, B-cell-specific CD30 expression led to a normal B-cell phenotype in young mice, but most aged mice exhibited significant expansion of B cells, T cells and myeloid cells and increased percentages of GC B cells and IgG1-switched cells. This may be driven by the expansion of CD4+ senescence-associated T cells and T follicular helper cells, which partially express CD30-L (CD153) and may stimulate CD30-expressing B cells. Inducing CD30 expression in antigen-activated B cells accelerates the GC reaction and augments plasma cell differentiation, possibly through the posttranscriptional upregulation of CXCR4. Furthermore, CD30 expression in GC B cells promoted the expansion of IgG1-switched cells, which displayed either a GC or memory-like B-cell phenotype, with abnormally high IgG1 levels compared with those in controls. These findings shed light on the role of CD30 signaling in GC B cells and suggest that elevated CD30+ B-cell numbers lead to pathological lymphocyte activation and proliferation.
© 2024. The Author(s).
-
IHC
-
Mus musculus (House mouse)
-
Immunology and Microbiology
In IScience on 15 November 2024 by Nguyen, L. T., Zimmermann, K., et al.
The transcription factor CCAAT enhancer binding protein alpha (C/EBPα) is a master regulator of myelopoiesis. CEBPA encodes a long (p42) and a truncated (p30) protein isoform from a single mRNA. Mutations that abnormally enhance expression of p30 are associated with acute myelogenous leukemia (AML). We show by mutational analysis that three highly conserved arginine residues in the p30 C/EBPα N-terminus, previously found to be methylated, are involved in myeloid lineage commitment, progenitor proliferation, and differentiation. The conservative amino acid substitution with lysine that retains the amino acid side chain charge enhanced progenitor proliferation, while a non-conservative substitution with uncharged side chains (alanine, leucine) impaired proliferation and enhanced granulopoiesis. Analysis of protein interactions suggested that arginine methylation of p30 C/EBPα differentially determines interactions with SWI/SNF and MLL complexes. Pharmacological targeting of p30 C/EBPα arginine methylation may have clinical relevance in myeloproliferative and inflammatory diseases, in neutropenia, and in leukemic stem cells.
© 2024 The Author(s).
In Nucleic Acids Research on 11 November 2024 by Bruzeau, C., Martin, O., et al.
In B lymphocytes, class switch recombination (CSR) is an essential process that adapts immunoglobulin (Ig) subtypes to antigen response. Taking place within the Ig heavy chain (IgH) locus, CSR needs controlled transcription of targeted regions governed by the IgH 3' regulatory region (3'RR). This super-enhancer is composed of four core enhancers surrounded by inverted repeated sequences, forming a quasi-palindrome. In addition to transcription, nuclear organization appears to be an important level in CSR regulation. While it is now established that chromatin loop extrusion takes place within IgH locus to facilitate CSR by bringing the donor and acceptor switch regions closer together, the underlying mechanism that triggers CSR loop formation remains partially understood. Here, by combining DNA 3D fluorescence in situhybridization with various high-throughput approaches, we deciphered critical functions for the 3'RR core enhancer element in nuclear addressing, accessibility and chromatin looping of the IgH locus. We conclude that the 3'RR core enhancers are necessary and sufficient to pre-organize the position and conformation of IgH loci in resting B-cell nuclei to enable the deletional recombination events required for productive successful CSR in activated B-cell nuclei.
© The Author(s) 2024. Published by Oxford University Press on behalf of Nucleic Acids Research.
-
Biochemistry and Molecular biology
Safety Evaluations of Rapamycin Perfluorocarbon Nanoparticles in Ovarian Tumor-Bearing Mice.
In Nanomaterials (Basel, Switzerland) on 31 October 2024 by Zhou, Q., Harding, J. C., et al.
Nanomedicine holds great potential for revolutionizing medical treatment. Ongoing research and advancements in nanotechnology are continuously expanding the possibilities, promising significant advancements in healthcare. To fully harness the potential of nanotechnology in medical applications, it is crucial to conduct safety evaluations for the nanomedicines that offer effective benefits in the preclinical stage. Our recent efficacy studies indicated that rapamycin perfluorocarbon (PFC) nanoparticles showed promise in mitigating cisplatin-induced acute kidney injury (AKI). As cisplatin is routinely administered to ovarian cancer patients as their first-line chemotherapy, in this study, we focused on evaluating the safety of rapamycin PFC nanoparticles in mice bearing ovarian tumor xenografts. Specifically, this study evaluated the effects of repeat-dose rapamycin PFC nanoparticle treatment on vital organs, the immune system, and tumor growth and assessed pharmacokinetics and biodistribution. Our results indicated that rapamycin PFC nanoparticle treatment did not cause any detectable adverse effects on cardiac, renal, or hepatic functions or on splenocyte populations, but it reduced the splenocyte secretion of IL-10, TNFα, and IL12p70 upon IgM stimulation. The pharmacokinetics and biodistribution results revealed a significant enhancement in the delivery of rapamycin to tumors by rapamycin PFC nanoparticles, which, in turn, led to a significant reduction in ovarian tumor growth. Therefore, rapamycin PFC nanoparticles have the potential to be clinically beneficial in cisplatin-treated ovarian cancer patients.
-
Cancer Research
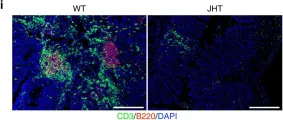
In Nat Commun on 25 April 2018 by Wunderlich, C. M., Ackermann, P. J., et al.
Fig.6.I

-
IHC-IF
-
Mus musculus (House mouse)
Collected and cropped from Nat Commun by CiteAb, provided under a CC-BY license
Image 1 of 4
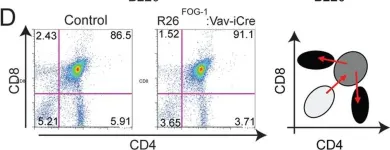
In PLoS One on 22 April 2014 by Du Roure, C., Versavel, A., et al.
Fig.7.D

-
FC/FACS
-
Collected and cropped from PLoS One by CiteAb, provided under a CC-BY license
Image 1 of 4
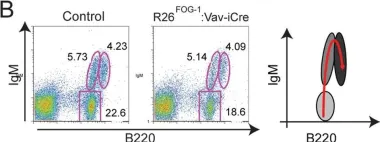
In PLoS One on 22 April 2014 by Du Roure, C., Versavel, A., et al.
Fig.7.B

-
FC/FACS
-
Collected and cropped from PLoS One by CiteAb, provided under a CC-BY license
Image 1 of 4
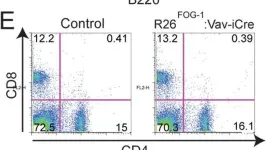
In PLoS One on 22 April 2014 by Du Roure, C., Versavel, A., et al.
Fig.7.E

-
FC/FACS
-
Collected and cropped from PLoS One by CiteAb, provided under a CC-BY license
Image 1 of 4