Neutrophils are highly abundant innate immune cells that are constantly produced from myeloid progenitors in the bone marrow. Differentiated neutrophils can perform an arsenal of effector functions critical for host defense. This study aims to quantitatively understand neutrophil mitochondrial metabolism throughout differentiation and activation, and to elucidate the impact of mitochondrial metabolism on neutrophil functions. To study metabolic remodeling throughout neutrophil differentiation, murine ER-Hoxb8 myeloid progenitor-derived neutrophils and human induced pluripotent stem cell-derived neutrophils were assessed as models. To study the metabolic remodeling upon neutrophil activation, differentiated ER-Hoxb8 neutrophils and primary human neutrophils were activated with various stimuli, including ionomycin, MSU crystals, and PMA. Characterization of cellular metabolism by isotopic tracing, extracellular flux analysis, metabolomics, and fluorescence-lifetime imaging microscopy revealed dynamic changes in mitochondrial metabolism. As neutrophils mature, mitochondrial metabolism decreases drastically, energy production is fully offloaded from oxidative phosphorylation, and glucose oxidation through TCA cycle is substantially reduced. Nonetheless, mature neutrophils retain the capacity for mitochondrial metabolism. Upon stimulation with certain stimuli, TCA cycle is rapidly activated. Mitochondrial pyruvate carrier inhibitors reduce this re-activation of the TCA cycle and inhibit the release of neutrophil extracellular traps. Mitochondrial metabolism also impacts neutrophil redox status, migration, and apoptosis without significantly changing overall bioenergetics. Together, these results demonstrate that mitochondrial metabolism is dynamically remodeled and plays a significant role in neutrophil function and fate. Furthermore, these findings point to the therapeutic potential of mitochondrial pyruvate carrier inhibitors in a range of conditions where dysregulated neutrophil response drives inflammation and contributes to pathology.
Product Citations: 124
Preprint on BioRxiv : the Preprint Server for Biology on 8 February 2025 by Lika, J., Votava, J. A., et al.
-
Biochemistry and Molecular biology
-
Cell Biology
In EBioMedicine on 1 February 2025 by Fournier, C., Mercey-Ressejac, M., et al.
mRNA-based cancer vaccines show promise in triggering antitumour immune responses. To combine them with existing immunotherapies, the intratumoral immune microenvironment needs to be deeply characterised. Here, we test nanostructured lipid carriers (NLCs), the so-called Lipidots®, for delivering unmodified mRNA encoding Ovalbumin (OVA) antigen to elicit specific antitumour responses.
We evaluated whether NLC OVA mRNA complexes activate dendritic cells (DCs) in vitro and identified the involved signalling pathways using specific inhibitors. We tested the anti-tumoral impact of Ova mRNA vaccine in B16-OVA and E.G7-OVA cold tumour-bearing C57Bl6 female mice as well as its synergy effect with an anti-PD-1 therapy by following the tumour growth and performing immunophenotyping of innate and adaptive immune cells. The intratumoral vaccine-related gene signature was assessed by RNA-sequencing. The immune memory response was assessed by re-challenging surviving treated mice with tumour cells.
Our vaccine activates DCs in vitro through the TLR4/8 and ROS signalling pathways and induces specific T cell activation while exhibits significant preventive and therapeutic antitumour efficacy in vivo. A profound intratumoral remodelling of the innate and adaptive immunity in association with an increase in the gene expression of chemokines (Cxcl10, Cxcl11, Cxcl9) involved in CD8+ T cell attraction were observed in immunised mice. The combination of vaccine and anti-PD-1 therapy improves the rates of complete responses and memory immune responses compared to monotherapies.
Lipidots® are effective platform for the development of vaccines against cancer based on mRNA delivery. Their combination with immune checkpoint blockers could counter tumour resistance and promote long-term antitumour immunity.
This work was supported by Inserm Transfert, la Région Auvergne Rhône Alpes, FINOVI, and the French Ministry of Higher Education, research and innovation (LipiVAC, COROL project, funding reference N° 2102992411).
Copyright © 2025 The Authors. Published by Elsevier B.V. All rights reserved.
-
Cancer Research
-
Genetics
-
Immunology and Microbiology
In The Journal of Clinical Investigation on 21 January 2025 by Cui, X., Hou, L., et al.
The bone marrow (BM) niche is critical in regulating hematopoiesis, and sexual dimorphism and its underlying mechanism in the BM niche and its impact on hematopoiesis are not well understood. We show that male mice exhibited a higher abundance of leptin-receptor-expressing mesenchymal stromal cells (LepR-MSCs) compared with female mice. Sex-mismatched coculture and BM transplantation showed that the male BM niche provided superior support for in vitro colony formation and in vivo hematopoietic engraftment. The cotransplantation of male stromal cells significantly enhanced engraftment in female recipients. Single-cell RNA-seq revealed that the lower expression of the X-linked lysine H3K4 demethylase, Kdm5c, in male MSCs led to the increased expression of Cxcl12. In MSC-specific Kdm5c-KO mouse model, the reduction of KDM5C in female MSCs enhanced MSC quantity and function, ultimately improving engraftment to the male level. Kdm5c thus plays a role in driving sexual dimorphism in the BM niche and hematopoietic regeneration. Our study unveils a sex-dependent mechanism governing the BM niche regulation and its impact on hematopoietic engraftment. The finding offers potential implications for enhancing BM transplantation efficacy in clinical settings by harnessing the resource of male MSCs or targeting Kdm5c.
PTEN acts as a crucial inflammatory checkpoint controlling TLR9/IL-6 axis in B cells.
In IScience on 19 July 2024 by Tsai, P. J., Chen, M. Y., et al.
Phosphatase and tensin homolog (PTEN) is vital for B cell development, acting as a key negative regulator in the PI3K signaling pathway. We used CD23-cre to generate PTEN-conditional knockout mice (CD23-cKO) to examine the impact of PTEN mutation on peripheral B cells. Unlike mb1-cre-mediated PTEN deletion in early B cells, CD23-cKO mutants exhibited systemic inflammation with increased IL-6 production in mature B cells upon CpG stimulation. Inflammatory B cells in CD23-cKO mice showed elevated phosphatidylinositol 3-phosphate [PI(3)P] levels and increased TLR9 endosomal localization. Pharmacological inhibition of PI(3)P synthesis markedly reduced TLR9-mediated IL-6. Single-cell RNA-sequencing (RNA-seq) revealed altered endocytosis, BANK1, and NF-κB1 expression in PTEN-deficient B cells. Ectopic B cell receptor (BCR) expression on non-inflammatory mb1-cKO B cells restored BANK1 and NF-κB1 expression, enhancing TLR9-mediated IL-6 production. Our study highlights PTEN as a crucial inflammatory checkpoint, regulating TLR9/IL-6 axis by fine-tuning PI(3)P homeostasis. Additionally, BCR downregulation prevents the differentiation of inflammatory B cells in PTEN deficiency.
© 2024 The Author(s).
-
Mus musculus (House mouse)
-
Immunology and Microbiology
GM-CSF-dependent CD301b+ lung dendritic cells confer tolerance to inhaled allergens
Preprint on Research Square on 4 June 2024 by Nakano, H., Wilkinson, C., et al.
Abstract The severity of allergic asthma is driven by the balance between allergen-specific T regulatory (Treg) and T helper (Th)2 cells. However, it is unclear whether specific subsets of conventional dendritic cells (cDCs) promote the differentiation of these two T cell lineaeges. We have identified a subset of lung resident type 2 cDCs (cDC2s) that display high levels of CD301b and have potent Treg-inducing activity ex vivo. Single cell RNA sequencing and adoptive transfer experiments show that during allergic sensitization, many CD301b+ cDC2s transition in a stepwise manner to CD200+ cDC2s that selectively promote Th2 differentiation. GM-CSF augments the development and maintenance of CD301b+ cDC2s in vivo, and also selectively expands Treg-inducing CD301b+ cDC2s derived from bone marrow. Upon their adoptive transfer to recipient mice, lung-derived CD301b+ cDC2s confer immunological tolerance to inhaled allergens. Thus, GM-CSF maintains lung homeostasis by increasing numbers of Treg-inducing CD301b+ cDC2s.
-
Immunology and Microbiology
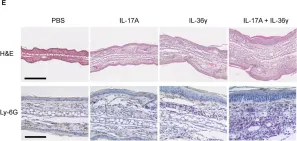
In Life (Basel) on 19 August 2021 by Kluwig, D., Huth, S., et al.
Fig.1.E

-
IHC
-
Collected and cropped from Life (Basel) by CiteAb, provided under a CC-BY license
Image 1 of 1