Hepatic fibrosis, ultimately causing hepatic sclerosis, remains significant health concerns. Adipose-derived mesenchymal stem cell (ADMSC)-derived exosomes (Exo) exhibit amelioration of liver injury. Hepatocyte growth factor (HGF) regulates hepatocyte growthn. However, its involvement during hepatic fibrosis remains unclear.
Isolation of ADMSCs and Exo, transfection of HGF overexpression, and activation of hepatic stellate cells (HSCs) by Angiotensin II (AngII) were conducted. Cells were randomized into HSC, AngII-HSC, ADMSCs-Exo, ADMSCsblank-Exo, and ADMSCsHGF-Exo, DPI, LY294002, and SB203580 groups. MTT for cell viability, cell migration, and flow cytometry for ROS were performed. BALB/c mice were treated with CCL4 for hepatic fibrosis models. The mice were randomized into Control, PBS, ADMSCs-Exo, ADMSCsblank-Exo, and ADMSCsHGF-Exo groups (n=6). HE, Sirius red, and Oil Red O staining, liver function indicators, and ELISA for oxidative stress were performed. ROS generation-related and PI3K/Akt/P38MAPK-related factors were detected by immunofluorescence, immunohistochemistry, and western blot.
After identification of ADMSC-Exo and transfection, AngII increased cell viability, migration, Collagen I (CoLI), α-smooth muscle actin (α-SMA), ROS, NADPH oxidase 4 (NOX4), PI3K, p-Akt, p-P38MAPK, ras-related C3 botulinum toxin substrate 1 (RAC1), p47phox, and p22phox expression. However, ADMSCsHGF-Exo, DPI, LY294002, and SB203580 reversed the above effects. Moreover, ADMSCsHGF-Exo inhibited pathological damage, fibrosis, lipid accumulation, ALT, AST, TBIL, CoLI, α-SMA, NOX4, MDA, PI3K, P-Akt, and P-P38MAPK expression, and increased ALB, SOD, GPx, CAT, GSH, Mn-SOD, Na+-K+-ATPase, and Ca2+-Mg2+-ATPase levels in hepatic fibrosis mice.
ADMSCsHGF-Exo attenuated hepatic fibrosis by inhibiting oxidative stress through activating the PI3K/Akt/P38MAPK pathway, providing valuable insights for potential treatment of liver fibrosis.
©The Author(s) 2024. Open Access. This article is licensed under a Creative Commons CC-BY International License.
Product Citations: 207
In Histology and Histopathology on 1 May 2025 by Zhou, H., Wu, Y., et al.
-
Pathology
The Role of N-acetylcysteine Amide in Acute Graft-versus-host Disease Mouse Model
Preprint on BioRxiv : the Preprint Server for Biology on 11 April 2025 by He, R., Zheng, W., et al.
Graft-versus-host disease (GvHD) remains one of the major complications following allogeneic hematopoietic cell transplantation (allo-HCT), resulting in reduced quality of life, morbidity, and mortality in transplanted patients. Clinical strategies to prevent GvHD are frequently associated with off-target effects and dose-related toxicity. Given that oxidative stress is elevated in allo-HCT recipients and contributes to the pathogenesis of GvHD, the current study aims to characterize the role of N-acetylcysteine amide (NACA), a novel antioxidant, as the prophylactic treatment for acute GvHD. Using a murine GvHD model, we found that oral administration of NACA significantly reduced GvHD severity, prolonged survival, and improved the clinical manifestations and integrity of target organs compared to saline or N-acetylcysteine (NAC) treatment. NACA modulated splenic T cells differentiation with an increase in the regulatory (T reg ) subset and a decrease in the cytotoxic (CD8 + ) subset. Moreover, inflammatory mediators, such as ROS and pro-inflammatory cytokines were downregulated by NACA treatment. In addition, NACA hindered donor T-cell proliferation in the recipients, and restrained Th1 and Th17, but not Th2 polarization. Importantly, NACA did not influence full donor engraftment in bone marrow and spleen. Taken together, our findings provide a new candidate for GvHD prophylactic treatment by targeting oxidative stress that can be easily translated to clinical use. Key Points NACA provides superior prophylactic effect against aGvHD compared to NAC in an allogeneic transplantation mouse model. NACA treatment neither showed systemic toxicity nor altered the engraftment of the donor cells.
The landscape of cell regulatory and communication networks in the human dental follicle.
In Frontiers in Bioengineering and Biotechnology on 20 February 2025 by Liu, J. N., Tian, J. Y., et al.
The dental follicle localizes the surrounding enamel organ and dental papilla of the developing tooth germ during the embryonic stage. It can differentiate and develop to form the periodontal ligament, cementum, and alveolar bone tissues. Postnatally, the dental follicle gradually degenerates, but some parts of the dental follicle remain around the impacted tooth. However, the specific cellular components and the intricate regulatory mechanisms governing the postnatal development and biological function of the dental follicle have not been completely understood.
We analyzed dental follicles with single-cell RNA sequencing (scRNA-seq) to reveal their cellular constitution molecular signatures by cell cycle analysis, scenic analysis, gene enrichment analysis, and cell communication analysis.
Ten cell clusters were identified with differential characteristics, among which immune and vessel-related cells, as well as a stem cell population, were revealed as the main cell types. Gene regulatory networks (GRNs) were established and defined four regulon modules underlying dental tissue development and microenvironmental regulation, including vascular and immune responses. Cell-cell communication analysis unraveled crosstalk between vascular and immune cell components in orchestrating dental follicle biological activities, potentially based on COLLAGAN-CD44 ligand-receptor pairs, as well as ANGPTL1-ITGA/ITGB ligand-receptor pairs.
We establish a landscape of cell regulatory and communication networks in the human dental follicle, providing mechanistic insights into the cellular regulation and interactions in the complex dental follicle tissue microenvironment.
Copyright © 2025 Liu, Tian, Liu, Cao, Lei, Zhang, Zhang, He, Zheng, Ma, Bai, Sui, Jin and Chen.
-
IHC-IF
Local Exosome Inhibition Potentiates Mild Photothermal Immunotherapy Against Breast Cancer.
In Advanced Science (Weinheim, Baden-Wurttemberg, Germany) on 1 January 2025 by Chen, Q., Li, Y., et al.
Limited immune infiltration within the tumor microenvironment (TME) hampers the efficacy of immune checkpoint blockade (ICB) therapy. To enhance immune infiltration, mild photothermal therapy (PTT) is often combined with immunotherapy. However, the impact of mild PTT on the TME remains unclear. The bioinformatics analyses reveal that mild PTT amplifies immune cell infiltration and stimulates T-cell activity. Notably, it accelerates the release of tumor cell-derived exosomes (TEX) and upregulates PD-L1 expression on both tumor cells and TEX. Consequently, it is proposed that locally inhibiting TEX release is crucial for overcoming the adverse effects of mild PTT, thereby enhancing ICB therapy. Thus, a multi-stage drug delivery system is designed that concurrently delivers photosensitizers (reduced graphene oxide nanosheets, NRGO), anti-PD-L1 antibodies, and exosome inhibitors (sulfisoxazole). The system employs a temperature-sensitive lipid gel as the primary carrier, with NRGO serving as a secondary carrier that supports photothermal conversion and incorporation of sulfisoxazole. Importantly, controlled drug release is achieved using near-infrared radiation. The findings indicate that this local combination therapy remodels the immunosuppressive TME through exosome inhibition and enhanced immune cell infiltration, while also boosting T-cell activity to trigger systemic antitumor immunity, showcasing the remarkable efficacy of this combination strategy in eradicating cold tumors.
© 2024 The Author(s). Advanced Science published by Wiley‐VCH GmbH.
-
Cancer Research
-
Immunology and Microbiology
In Oncoimmunology on 25 June 2024 by Harjunpää, H., Somermäki, R., et al.
Dendritic cells (DCs) are the main antigen presenting cells of the immune system and are essential for anti-tumor responses. DC-based immunotherapies are used in cancer treatment, but their functionality is not optimized and their clinical efficacy is currently limited. Approaches to improve DC functionality in anti-tumor immunity are therefore required. We have previously shown that the loss of β2-integrin-mediated adhesion leads to epigenetic reprogramming of bone marrow-derived DCs (BM-DCs), resulting in an increased expression of costimulatory markers (CD86, CD80, and CD40), cytokines (IL-12) and the chemokine receptor CCR7. We now show that the loss of β2-integrin-mediated adhesion of BM-DCs also leads to a generally suppressed metabolic profile, with reduced metabolic rate, decreased ROS production, and lowered glucose uptake in cells. The mRNA levels of glycolytic enzymes and glucose transporters were reduced, indicating transcriptional regulation of the metabolic phenotype. Surprisingly, although signaling through a central regulator of immune cell metabolisms, the mechanistic target of rapamycin (mTOR), was increased in BM-DCs with dysfunctional integrins, rapamycin treatment revealed that mTOR signaling was not involved in suppressing DC metabolism. Instead, bioinformatics and functional analyses showed that the Ikaros transcription factor may be involved in regulating the metabolic profile of non-adhesive DCs. Inversely, we found that induction of metabolic stress through treatment of cells with low levels of an inhibitor of glycolysis, 2-deoxyglucose (2DG), led to increased BM-DC activation. Specifically, 2DG treatment led to increased levels of Il-12 and Ccr7 mRNA, increased production of IL-12, increased levels of cell surface CCR7 and increased in vitro migration and T cell activation potential. Furthermore, 2DG treatment led to increased histone methylation in cells (H3K4me3, H3K27me3), indicating metabolic reprogramming. Finally, metabolic stress induced by 2DG treatment led to improved BM-DC-mediated anti-tumor responses in vivo in a melanoma cancer model, B16-OVA. In conclusion, our results indicate a role for β2-integrin-mediated adhesion in regulating a novel type of metabolic reprogramming of DCs and DC-mediated anti-tumor responses, which may be targeted to enhance DC-mediated anti-tumor responses in cancer immunotherapy.
© 2024 The Author(s). Published with license by Taylor & Francis Group, LLC.
-
Mus musculus (House mouse)
-
Biochemistry and Molecular biology
-
Cancer Research
-
Cell Biology
-
Immunology and Microbiology
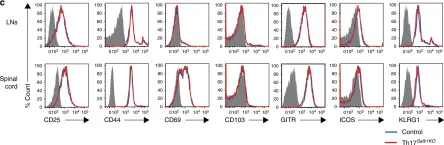
In Nat Commun on 15 December 2021 by Honda, M., Kadohisa, M., et al.
Fig.4.D

-
FC/FACS
-
Mus musculus (House mouse)
Collected and cropped from Nat Commun by CiteAb, provided under a CC-BY license
Image 1 of 8
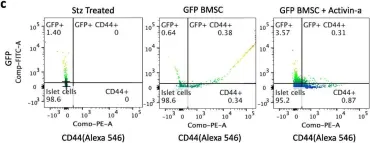
In Stem Cell Res Ther on 30 July 2020 by Dadheech, N., Srivastava, A., et al.
Fig.3.C

-
FC/FACS
-
Mus musculus (House mouse)
Collected and cropped from Stem Cell Res Ther by CiteAb, provided under a CC-BY license
Image 1 of 8
In Nat Commun on 1 February 2019 by Yasuda, K., Kitagawa, Y., et al.
Fig.3.C

-
FC/FACS
-
Mus musculus (House mouse)
Collected and cropped from Nat Commun by CiteAb, provided under a CC-BY license
Image 1 of 8
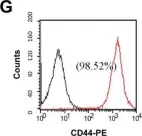
In PLoS One on 17 September 2013 by Wen, S. T., Chen, W., et al.
Fig.1.G

-
FC/FACS
-
Collected and cropped from PLoS One by CiteAb, provided under a CC-BY license
Image 1 of 8
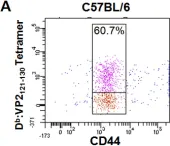
In PLoS One on 30 August 2008 by Suidan, G. L., McDole, J. R., et al.
Fig.1.A

-
FC/FACS
-
Collected and cropped from PLoS One by CiteAb, provided under a CC-BY license
Image 1 of 8
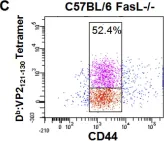
In PLoS One on 30 August 2008 by Suidan, G. L., McDole, J. R., et al.
Fig.1.C

-
FC/FACS
-
Collected and cropped from PLoS One by CiteAb, provided under a CC-BY license
Image 1 of 8
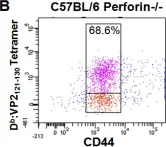
In PLoS One on 30 August 2008 by Suidan, G. L., McDole, J. R., et al.
Fig.1.B

-
FC/FACS
-
Collected and cropped from PLoS One by CiteAb, provided under a CC-BY license
Image 1 of 8
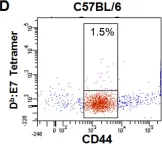
In PLoS One on 30 August 2008 by Suidan, G. L., McDole, J. R., et al.
Fig.1.D

-
FC/FACS
-
Collected and cropped from PLoS One by CiteAb, provided under a CC-BY license
Image 1 of 8