B lymphopoiesis is orchestrated by lineage-specific transcription factors. In B cell progenitors, lineage commitment is mediated by Pax5, which is commonly mutated in B cell acute lymphoblastic leukemia. Despite its essential role in immunity, the mechanisms regulating Pax5 function remain largely unknown. Here, we found that the NAD+-dependent enzyme SIRT7 coordinates B cell development through deacetylation of Pax5 at K198, which promotes Pax5 protein stability and transcriptional activity. Neither Pax5K198 deacetylated nor acetylated mimics rescued B cell differentiation in Pax5-/- pro-B cells, suggesting that B cell development requires Pax5 dynamic deacetylation. The Pax5K198 deacetylation mimic restored lineage commitment in Pax5-/- pro-B cells and B cell differentiation in Sirt7-/- pro-B cells, suggesting the uncoupling of differentiation from lineage commitment. The SIRT7-Pax5 interplay was conserved in B cell acute lymphoblastic leukemia, where SIRT7 expression correlated with good prognosis. Our findings reveal a crucial mechanism for B lymphopoiesis and highlight the relevance of sirtuins in immune function.
© 2024. The Author(s).
Product Citations: 26
In Nature Immunology on 1 December 2024 by Gámez-García, A., Espinosa-Alcantud, M., et al.
-
Immunology and Microbiology
Regulation of Leucine-Rich Repeat Kinase 2 by inflammation and IL-4
Preprint on BioRxiv : the Preprint Server for Biology on 2 May 2024 by Dikovskaya, D., Pemberton, R., et al.
Summary Mutations in Leucine-Rich Repeat protein Kinase 2 (LRRK2) are associated with Parkinson’s disease (PD) and Crohn’s disease (CD), but the regulation of LRRK2 during inflammation remains relatively unexplored. Here we developed a flow cytometry-based assay to assess LRRK2 activity in individual cells and created an EGFP-LRRK2-knock-in reporter mouse to analyse cell-specific LRRK2 expression. Using these tools, we catalogued LRRK2 level and activity in splenic and intestinal tissues. Inflammation increased LRRK2 expression and activity in B-cells, immature neutrophils and immature monocytes, but decreased these in dendritic cells and eosinophils. In mature neutrophils, inflammation stimulated LRRK2 activity but reduced EGFP-LRRK2 level. A kinase-activating PD-associated R1441C-LRRK2 mutation exacerbated inflammation-induced activation of LRRK2 specifically in monocytes and macrophages without affecting LRRK2 levels. Finally, we identified IL-4 as a novel factor that upregulated LRRK2 expression and activity in B-cells in vitro , replicating the inflammatory effects observed in vivo . Our findings provide valuable new insights into the regulation of the LRRK2 pathway in immune cells, crucial for understanding LRRK2 and its therapeutic potential in inflammatory diseases such as CD.
-
Mus musculus (House mouse)
-
Immunology and Microbiology
Preprint on Research Square on 13 July 2023 by Rivera-Correa, J., Gupta, S., et al.
Protective humoral responses require that B cells successfully complete their differentiation programs even when exposed to hostile environments generated during severe infections, like the massive hemolysis triggered by malaria. The mechanisms utilized by differentiating B cells to withstand damaging conditions replete in PAMPs and DAMPs are poorly understood. Here we demonstrate that the serine-threonine kinase ROCK1 enables B cells to execute their differentiation programs upon exposure to PAMPs and high levels of heme, a critical DAMP, by controlling two key heme-regulated molecules, Bach2 and HRI. ROCK1 restrains plasma cell differentiation by phosphorylating Bach2. As B cells differentiate in the presence of PAMPs and heme, furthermore, ROCK1 limits the proinflammatory potential of B cells and restrains mTORC1 activity by controlling the assembly of multimolecular complexes that contain the adaptor p62, raptor, ripoptosome components, and molecules involved in RNA metabolism and proteostasis. ROCK1 regulates formation of these complexes by controlling the interplay between HSPs and the stress kinase HRI. Thus, ROCK1 helps B cells cope with intense pathogen-driven destruction by coordinating the activity and localization of key molecules that mediate cell-fate decisions, effector functions, and RNA and protein homeostasis. These ROCK1-dependent mechanisms may be widely employed by cells to handle severe environmental stresses and these findings may be broadly relevant for infections, vaccine development, and immune-mediated diseases marked by chronic tissue damage like autoimmune disorders.
-
Mus musculus (House mouse)
-
Immunology and Microbiology
In Cell Reports on 14 June 2022 by Worth, A. N., Palmer, V. L., et al.
B1 B cells reactive to phosphatidyl choline (PtC) exhibit restricted immunoglobulin heavy chain (HC) and light chain (LC) combinations, exemplified by VH12/Vκ4/5H. Two checkpoints are thought to focus PtC+ B cell maturation in VH12-transgenic mice (VH12 mice): V-J rearrangements encoding a "permissive" LC capable of VH12 HC pairing are selected first, followed by positive selection based on PtC binding, often requiring LC receptor editing to salvage PtC- B cells and acquire PtC reactivity. However, evidence obtained from breeding VH12 mice to editing-defective dnRAG1 mice and analyzing LC sequences from PtC+ and PtC- B cell subsets instead suggests that receptor editing functions after initial positive selection to remove PtC+ B cells in VH12 mice. This offers a mechanism to constrain natural, polyreactive B cells to limit their frequency. Sequencing also reveals occasional in-frame hybrid LC genes, reminiscent of type 2 gene replacement, that, testing suggests, arise via a recombination-activating gene (RAG)-independent mechanism.
Copyright © 2022 The Author(s). Published by Elsevier Inc. All rights reserved.
-
Immunology and Microbiology
In Molecular and Cellular Biology on 22 November 2021 by Liu, R., King, A., et al.
Toll-like receptors (TLRs) and interleukin-1 (IL-1) receptors regulate immune and inflammatory responses by activating the NF-κB pathway. Here, we report that B-cell-specific loss of dynein light chain 1 (DYNLL1, LC8) or its designated transcription factor ASCIZ (ATMIN) leads to severely reduced in vivo antibody responses to TLR4-dependent but not T-cell-dependent antigens in mice. This defect was independent of DYNLL1's established roles in modulating BIM-dependent apoptosis and 53BP1-dependent antibody class-switch recombination. In B cells and fibroblasts, the ASCIZ-DYNLL1 axis was required for TLR4-, IL-1-, and CD40-mediated NF-κB pathway activation but dispensable for antigen receptor and tumor necrosis factor α (TNF-α) signaling. In contrast to previous reports that overexpressed DYNLL1 directly inhibits the phosphorylation and degradation of the NF-κB inhibitor IκBα, we found here that under physiological conditions, DYNLL1 is required for signal-specific activation of the NF-κB pathway upstream of IκBα. Our data identify DYNLL1 as a signal-specific regulator of the NF-κB pathway and indicate that it may act as a universal modulator of TLR4 (and IL-1) signaling with wide-ranging roles in inflammation and immunity.
-
FC/FACS
-
Mus musculus (House mouse)
-
Cell Biology
In Front Immunol on 7 April 2018 by Liu, R., King, A., et al.
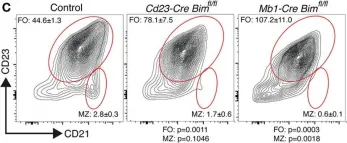
Fig.2.C

-
FC/FACS
-
Mus musculus (House mouse)
Collected and cropped from Front Immunol by CiteAb, provided under a CC-BY license
Image 1 of 3
In Front Immunol on 7 April 2018 by Liu, R., King, A., et al.
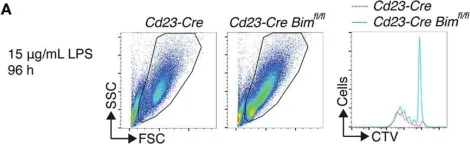
Fig.3.A

-
FC/FACS
-
Mus musculus (House mouse)
Collected and cropped from Front Immunol by CiteAb, provided under a CC-BY license
Image 1 of 3
In Front Immunol on 7 April 2018 by Liu, R., King, A., et al.
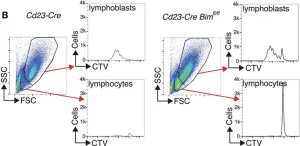
Fig.3.B

-
FC/FACS
-
Mus musculus (House mouse)
Collected and cropped from Front Immunol by CiteAb, provided under a CC-BY license
Image 1 of 3