Herein, we report a microenvironment self-adaptive multifunctional hydrogel (GBOD-PF) dressing with intrinsic hemostasis, antimicrobial and anti-inflammatory properties that promotes collagen deposition, activates the immune response, and angiogenesis, accelerates large-scale chronic wound healing. To address these needs, aldehyde-functionalized dextran (ODT) was first synthesized through oxidation, and an injectable hydrogel was prepared by mixing Gel and ODT solutions in the presence of borax and paeoniflorin (PF) through the formation of dynamic Schiff base bonds (GBOD-PF). This multifunctional hydrogel exhibits remodeling and self-healing properties, enhanced adhesion strength and biocompatibility. Moreover, it possesses broad-spectrum antibacterial activity and superior hemostasis, providing early-stage protection for complex wounds. Notably, GBOD-PF targets the TLR4/NF-κB signaling pathway, a core mediator of inflammation, thereby dampening the inflammatory cascade. The results demonstrate the application of the GBOD-PF hydrogel in an infection wound and diabetic model wound healing model, which enhanced immune response, induced M1-to-M2 macrophage repolarization to establish anti-inflammatory microenvironment, regulated MMP-9, promoted angiogenesis, thereby inducing a pro-regenerative response. Therefore, this work provides an effective strategy for the treatment of chronic wound repair and tissue regeneration.
© 2025 The Authors.
Product Citations: 2,509
In Materials Today. Bio on 1 December 2025 by Fang, J., Wang, Y., et al.
In Drug Delivery on 1 December 2025 by Nyesiga, B., Hägerbrand, K., et al.
Delivery of antigenic peptides to antigen presenting cells (APCs) such as dendritic cells (DCs) using monoclonal antibodies (mAbs) is an attractive approach to evoke antigen-specific T cell activation and improve drug efficacy. Peptide linkage to mAbs has previously been achieved through genetic fusion, chemical conjugation, nano-engineered platforms and high affinity peptides. In this study, we have developed a flexible antibody-peptide linking technology using oppositely charged coiled coil domains to non-covalently link peptides to mAbs. The technology comprises (1) an anti-CD40 mAb connected with negatively charged E domains and (2) an immunogenic OVA peptide (SIINFEKL) from ovalbumin used as a model antigenic peptide fused with positively charged K domains. Combining these constructs leads to the formation of complexes that can be targeted to CD40 expressed on cells. Proof of concept antibody constructs connected with E domains generated from transient expressions exhibited good manufacturability, binding, and stability attributes comparable to a control mAb. Also, optimal repeat lengths for coiled-coil oligomerization domains were identified in these studies. Binding kinetics studies showed that connecting E domains to mAbs do not impede Fc gamma and neonatal receptor interactions. Additionally, formation of stable complexes capable of binding CD40 expressing cells was demonstrated in vitro. In vivo functionality evaluations showed that treatment of human CD40 transgenic mice with complexes elicited expansion of OVA peptide-specific CD8+ T cells and potent antitumor effects superior to peptide monotherapies. Overall, these findings demonstrate that the technology has great potential for application as an in vivo tool for antigenic peptide delivery.
-
Immunology and Microbiology
In Bioactive Materials on 1 December 2025 by Xue, Y., Bai, S., et al.
Achieving robust and durable cellular immunity remains a key challenge in the development of subunit vaccines, primarily due to inefficient antigen cross-presentation and inadequate immune activation. Here, we engineered a series of nano-emulsions by conjugating human serum albumin (HSA) with fatty acids of varying chain lengths. Through systematic screening, the palmitic acid-modified nano-emulsion was identified as the most effective carrier, exhibiting intrinsic self-adjuvant properties and a strong capacity to elicit cellular immune responses. Notably, this formulation enables cascade-targeted delivery, trafficking sequentially from lymph nodes to antigen-presenting cells (APCs), and ultimately to the endoplasmic reticulum (ER). Upon co-delivery of the model antigen ovalbumin (OVA) and a stimulator of interferon genes (STING) agonist, the nano-emulsion facilitates both efficient antigen cross-presentation and precise intracellular activation of the STING pathway. This synergistic mechanism significantly enhances CD8+ T cell responses and promotes durable memory formation, resulting in potent antitumor efficacy in murine models. Collectively, this study presents a safe and versatile nano-emulsion platform that overcomes key barriers in subunit vaccine delivery, offering a promising strategy for next-generation vaccine design.
© 2025 The Authors.
-
Immunology and Microbiology
In Frontiers in Immunology on 24 October 2025 by Wiinberg, M., Melander, F., et al.
The Toll-like receptor (TLR) 7/8 agonist resiquimod has shown promise for precancerous lesions of cutaneous squamous cell carcinoma (cSCC) but remains unexplored as a treatment for cSCC. Additionally, ablative fractional laser (AFL) has been shown to enhance the efficacy of TLR7 agonist in mouse tumor models. This study investigates the efficacy of intratumoral resiquimod formulated into a sustained-release gel (RSQ-gel) in a cSCC mouse model and compares RSQ-gel with topical imiquimod (IMQ) cream, a clinically approved TLR7 agonist. We further examine whether adjuvant AFL enhances the efficacy of RSQ-gel.
A syngeneic transplanted cSCC mouse model was established using cells from a UVR-induced cSCC mouse model. The immunostimulatory effects of RSQ-gel were assessed by analyzing the expression of the activation marker CD86 on plasmacytoid dendritic cells (pDC) and cross-presenting conventional type I dendritic cells (XCR1+ cDC1) via flow cytometry. Tumor growth and survival outcomes were evaluated for RSQ-gel as monotherapy and in combination with AFL.
RSQ-gel was associated with activation of pDCs and XCR1+ cDC1s in the tumor-draining lymph node, as indicated by higher expression of CD86 compared to IMQ (P< 0.0001, P = 0.00175, respectively). RSQ-gel monotherapy delayed tumor growth but did not prolong survival (P = 0.0651). However, combining RSQ-gel with AFL resulted in prolonged survival compared to AFL-treated and untreated mice (P = 0.0153, P = 0.0214, respectively). Weekly RSQ-gel treatment induced comparable efficacy to daily topical IMQ treatment.
RSQ-gel with AFL demonstrated significant antitumor efficacy in the cSCC mouse model. Local RSQ-gel combined with adjuvant AFL may offer a promising therapeutic approach for cSCC.
Copyright © 2025 Wiinberg, Melander, Lerche, Andresen, Olesen and Haedersdal.
-
Cancer Research
In Commun Med (Lond) on 23 October 2025 by Harre, P., Hohenberger, K., et al.
Non-small cell lung cancer (NSCLC) is the leading cause of cancer mortality, and most patients fail to respond to immune checkpoint inhibitors (ICIs). Nutritional factors, particularly dietary lipids, influence cancer progression and immunity. Cholesterol can promote tumor growth and immune evasion, whereas ω-3 polyunsaturated fatty acids (PUFAs) may exert anti-inflammatory and immunostimulatory effects. We aimed to determine how dietary lipids influence NSCLC progression and whether ω-3 PUFA supplementation can enhance the efficacy of PD-1 blockade.
Serum lipid profiles from 152 NSCLC patients were analyzed for associations with prognosis. Female murine NSCLC models were fed diets enriched in cholesterol, saturated fats, or ω-3 PUFAs, with or without anti-PD-1 therapy. Tumor growth, immune infiltration, cytokine production, and epithelial-mesenchymal transition (EMT) markers were assessed by flow cytometry, ELISA, and gene expression analysis.
In patients, elevated serum triglycerides correlate with poor outcomes, and tumor cholesterol metabolism links to EMT marker expression. In female mice, cholesterol-rich diets accelerate tumor growth, increase EMT-associated genes (SNAIL, Vimentin), and elevate pro-tumoral cytokines (IL-6, IL-10, IL-17A). ω-3 PUFA diets reduce tumor burden, lower immunosuppressive cytokines, decrease regulatory T cells, and enhance cytotoxic T cell activity. Combining ω-3 PUFAs with anti-PD-1 therapy synergistically suppresses tumor growth and improves antitumor immune responses.
Dietary lipids modulate NSCLC progression via metabolic, inflammatory, and immune pathways. ω-3 PUFA supplementation counteracts cholesterol-driven tumor promotion and augments PD-1 blockade efficacy, supporting dietary modulation as a complementary strategy to improve immunotherapy outcomes in NSCLC.
© 2025. The Author(s).
-
Cancer Research
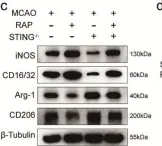
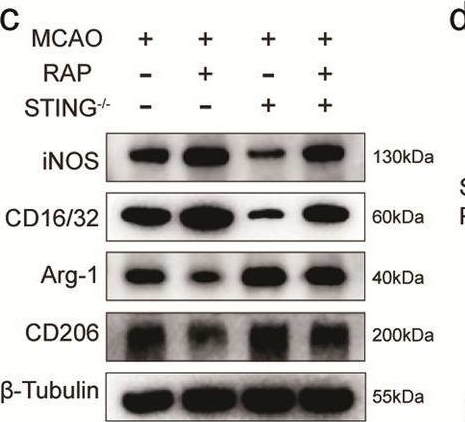
In Cell Death Dis on 13 November 2024 by Kong, L., Xu, P., et al.
Fig.7.C
-
WB
-
Mus musculus (House mouse)
Collected and cropped from Cell Death & Disease by CiteAb, provided under a CC-BY license
Image 1 of 10
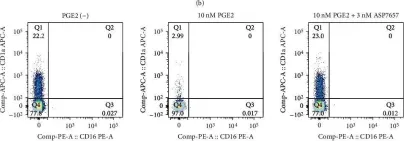
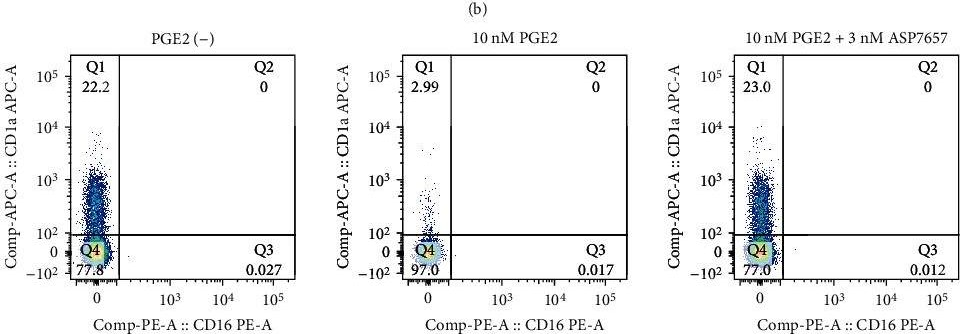
In Biomed Res Int on 7 December 2023 by Nishibata, T., Amino, N., et al.
Fig.1.B
-
FC/FACS
-
Homo sapiens (Human)
Collected and cropped from BioMed Research International by CiteAb, provided under a CC-BY license
Image 1 of 10
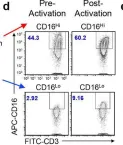
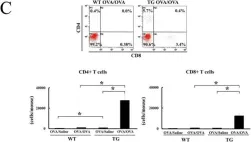
In Nat Commun on 8 November 2023 by Lee, D., Dunn, Z. S., et al.
Fig.1.D
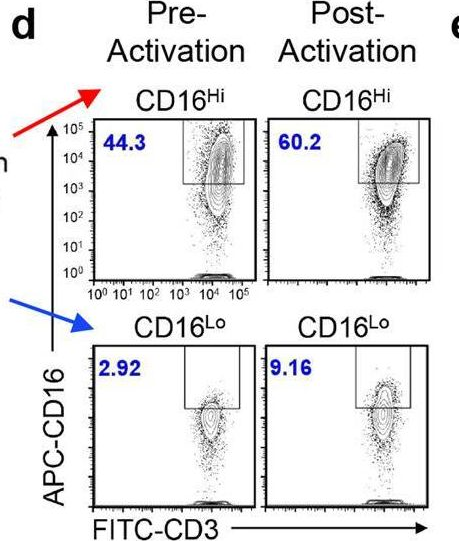
-
FC/FACS
-
Homo sapiens (Human)
Collected and cropped from Nature Communications by CiteAb, provided under a CC-BY license
Image 1 of 10
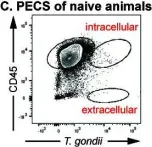
In MBio on 31 October 2023 by Vizcarra, E. A., Goerner, A. L., et al.
Fig.7.C
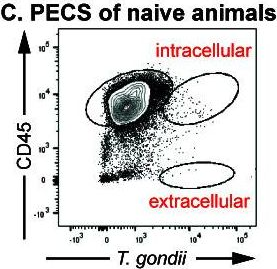
-
FC/FACS
-
Mus musculus (House mouse)
Collected and cropped from mBio by CiteAb, provided under a CC-BY license
Image 1 of 10
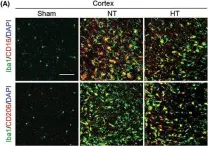
In CNS Neurosci Ther on 1 January 2023 by Liu, L., Liu, J., et al.
Fig.4.A
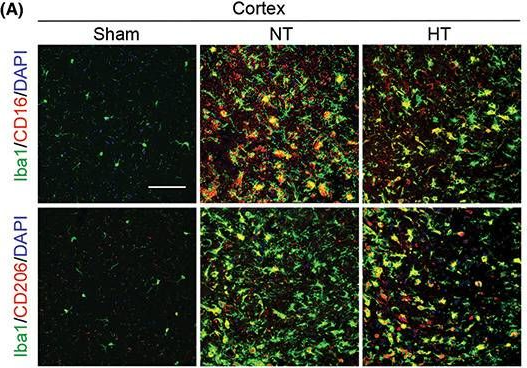
-
IHC-IF
-
Mus musculus (House mouse)
Collected and cropped from CNS Neuroscience Therapeutics by CiteAb, provided under a CC-BY license
Image 1 of 10
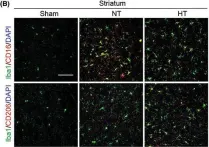
In CNS Neurosci Ther on 1 January 2023 by Liu, L., Liu, J., et al.
Fig.4.B
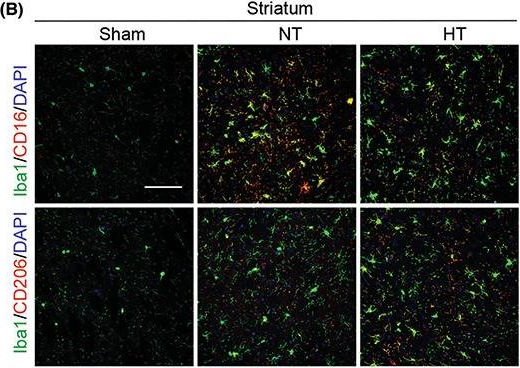
-
IHC-IF
-
Mus musculus (House mouse)
Collected and cropped from CNS Neuroscience Therapeutics by CiteAb, provided under a CC-BY license
Image 1 of 10
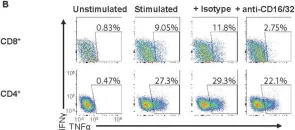
In PLoS Pathog on 1 September 2022 by Iyer, R. F., Edwards, D. M., et al.
Fig.5.B
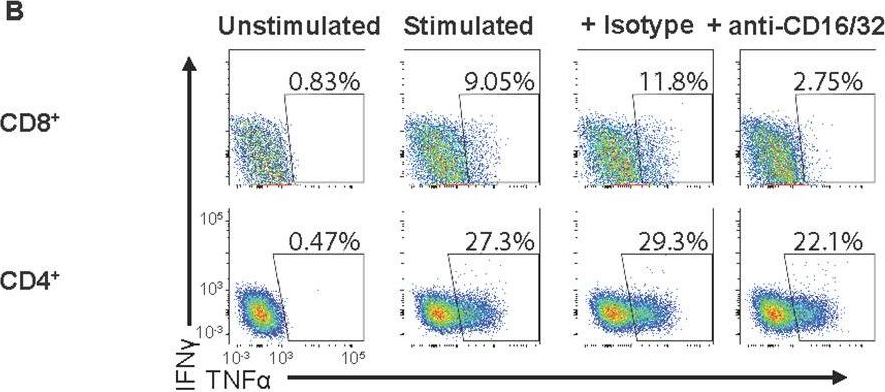
-
FC/FACS
-
Collected and cropped from PLoS Pathogens by CiteAb, provided under a CC-BY license
Image 1 of 10
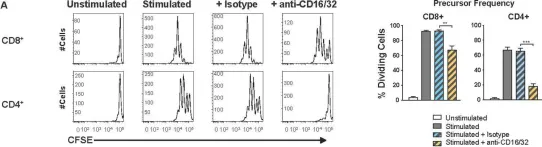
In PLoS Pathog on 1 September 2022 by Iyer, R. F., Edwards, D. M., et al.
Fig.5.A
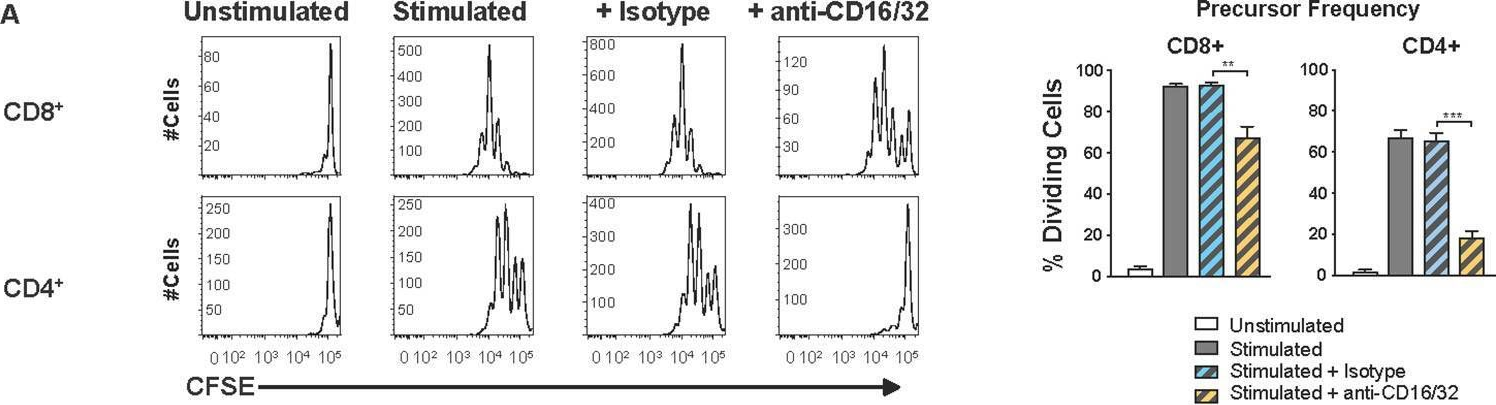
-
FC/FACS
-
Collected and cropped from PLoS Pathogens by CiteAb, provided under a CC-BY license
Image 1 of 10
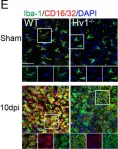
In J Neuroinflammation on 6 November 2020 by Chen, M., Yang, L. L., et al.
Fig.2.E
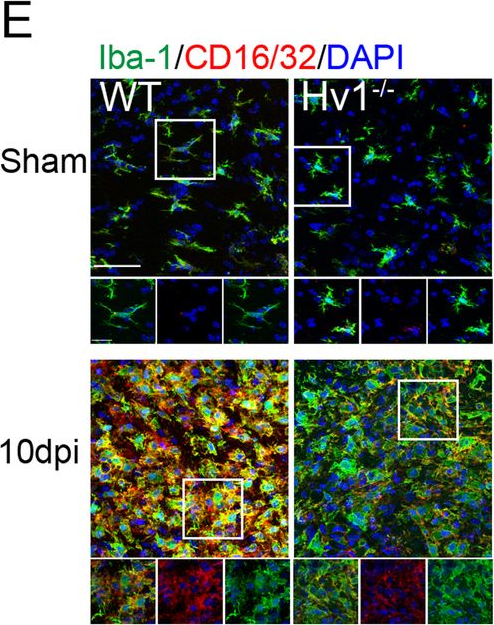
-
IHC
-
Mus musculus (House mouse)
Collected and cropped from Journal of Neuroinflammation by CiteAb, provided under a CC-BY license
Image 1 of 10
In PLoS One on 6 February 2013 by Sawada, M., Kawayama, T., et al.
Fig.2.C
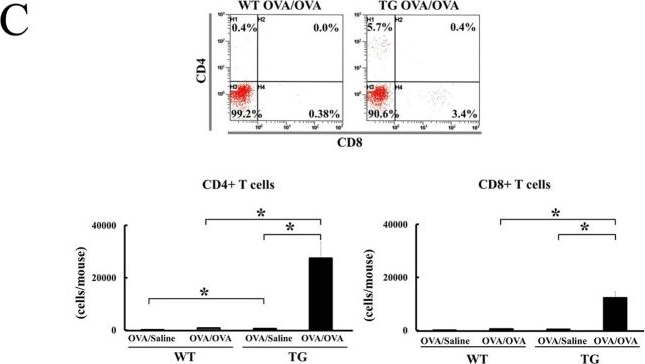
-
FC/FACS
-
Mus musculus (House mouse)
Collected and cropped from PLoS ONE by CiteAb, provided under a CC-BY license
Image 1 of 10