Achieving a cure is an urgent need for patients with advanced solid tumors. Here, we discover that oncolytic virus (OV) infection enhances IL-18 receptor expression but fails to increase IL-18 ligand expression. Therefore, we engineer armed oncolytic alphavirus M1 expressing wild-type IL-18 (wtIL-18) or a mutant variant (mutIL-18) that evades IL-18 binding protein (IL-18BP) while maintaining IL-18 receptor (IL-18R) binding. Intravenous administration of M1-mutIL-18 suppresses the growth of multiple advanced solid tumors in C57BL/6 and BALB/c mouse models and promotes long-term systemic immune memory. Mechanistically, armed M1-mutIL-18 enhances directed clonal expansion and differentiation of CD8+ T cells and sustains IFN-γ production. Thus, armed M1-mutIL-18 promotes dendritic cell (DC) activation, priming and activation of CD8+ T cells in lymphatic organs, and infiltration of IL-18R+ CD8+ T cells in the tumor microenvironment, establishing a positive feedback loop. We further show that a PD-L1 inhibitor enhances the anti-tumor efficacy of mutIL-18 OVs. These results highlight the importance of the IL-18 pathway in oncolytic virus therapy and implicate reprogramming ligand-receptor interaction as an effective strategy for immunotherapy.
© 2025. The Author(s).
Product Citations: 138
In Nature Communications on 3 July 2025 by Fan, Z., Liu, Y., et al.
RIPK1 ablation in T cells results in spontaneous enteropathy and TNF-driven villus atrophy.
In EMBO Reports on 1 May 2025 by Huysentruyt, J., Steels, W., et al.
RIPK1 is a crucial regulator of cell survival, inflammation and cell death. Human RIPK1 deficiency leads to early-onset intestinal inflammation and peripheral T cell imbalance, though its role in αβT cell-mediated intestinal homeostasis remains unclear. In this study, we demonstrate that mice with RIPK1 ablation in conventional αβT cells (Ripk1ΔCD4) developed a severe small intestinal pathology characterized by small intestinal elongation, crypt hyperplasia, and duodenum-specific villus atrophy. Using mixed bone marrow chimeras reveals a survival disadvantage of αβT cells compared to γδT cells in the small intestine. Broad-spectrum antibiotic treatment ameliorates crypt hyperplasia and prevents intestinal elongation, though villus atrophy persists. Conversely, crossing Ripk1ΔCD4 with TNF receptor 1 Tnfr1-/- knockout mice rescues villus atrophy but not intestinal elongation. Finally, combined ablation of Ripk1∆CD4 and Casp8∆CD4 fully rescues intestinal pathology, revealing that αβT cell apoptosis in Ripk1∆CD4 drives the enteropathy. These findings demonstrate that RIPK1-mediated survival of αβT cells is essential for proximal small intestinal homeostasis. In Ripk1∆CD4 mice, the imbalanced T cell compartment drives microbiome-mediated intestinal elongation and TNF-driven villus atrophy.
© 2025. The Author(s).
-
Immunology and Microbiology
In Journal for Immunotherapy of Cancer on 5 November 2024 by Degavre, C., Lepez, A., et al.
Immunogenic cell death (ICD) and ferroptosis have recently emerged as key factors in the anticancer immune response. Among the treatments able to induce ICD and the associated release of danger signals is photodynamic therapy (PDT). Ferroptosis for its part results from lipid peroxidation and is induced by CD8+ T cells to kill nearby cancer cells on IFN-γ production. We aimed to combine the two concepts, that is, to evaluate whether the strong pro-oxidant effects of PDT may promote ferroptosis and antigen release and to develop a procedure for in situ PDT to prepare the soil for highly endocytotic immature dendritic cell (iDC) adoptive transfer. This approach was implemented for managing peritoneal carcinomatosis, a lesion often associated with poor outcomes.
We used three-dimensional (3D) heterotypic spheroids made of cancer cells, exposed them to a white light-activated OR141 photosensitizer (PS), and subsequently complexified them by adding iDC and naive lymphocytes. We next used a model of mouse peritoneal carcinomatosis and administered PDT using laparoscopy to locally induce photoactivation using the endoscope light. The immune response following adoptive transfer of iDC was tracked both in vivo and ex vivo using isolated immune cells from in situ vaccinated mice.
Cancer cells undergoing PDT-induced cell death significantly increased ICD markers and the infiltration of iDCs in spheroids, relying on ferroptosis. These actions induced the sequential activation of CD8+ and CD4+ T cells as revealed by a significant spheroid 3D structure deterioration and, remarkably, were not recapitulated by conventional ferroptosis inducer RSL3. Using LED light from an endoscope for in situ photoactivation of PS enabled us to apply the vaccination modality in mice with peritoneal tumors. Consecutive intraperitoneal injection of iDCs resulted in delayed tumor growth, increased survival rates, and prevented tumor relapse on rechallenge. CD8+ T cell response was supported by depletion experiments, nodal detection of early activated T cells, and ex vivo T cell-induced cytotoxicity toward spheroids.
The combination of in situ PDT locally delivered by an endoscope light and iDC administration induces a durable memory immune response against peritoneal carcinomatosis thereby opening new perspectives for the treatment of a life-threatening condition.
© Author(s) (or their employer(s)) 2024. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
-
FC/FACS
-
Mus musculus (House mouse)
-
Immunology and Microbiology
Preprint on BioRxiv : the Preprint Server for Biology on 4 November 2024 by Zhang, D., Xiao, Q., et al.
There continues to be a dearth of competent inhalable mRNA delivery although it holds great potential for addressing a wide variety of refractory diseases. The huge advances seen with parenteral-administered lipid nanoparticle (LNP) have not been translated into nebulized mRNA delivery due to the aggressive nebulization process and insurmountable barriers inherent to respiratory mucosa. Here, we show amphiphilic block copolymers revealed by machine learning (ML) can spontaneously form stabilized nanoparticles (PoLixNano) with the lipids components of LNP and simultaneously impart the PoLixNano with "shield" (shear force-resistant) and "spear" (pulmonary barriers-penetrating abilities) capabilities. We present a ML approach that leverages physicochemical properties and inhaled mRNA transfection profiles of a chemically diverse library of polymeric components to validate the integration of "shield" and "spear" properties as highly predictive indicators of transfection efficiency. This quantitative structure-mRNA transfection prediction (QSMTP) model identifies top-performing amphiphilic-copolymers from more than 10000 candidates and suggests their mucus-penetrating ability outweights the shear force-resistant property in contributing to efficient mRNA transfection. The optimized PoLixNano substantially outperforms the LNP counterpart and mediates up to 1114-times higher levels of mRNA transfection in animal models with negligible toxicities. The PoLixNano promotes overwhelming SARS-CoV-2 antigen-specific sIgA antibody secretion and expansion of TRM cells which collectively confers 100% protection in mice against lethal SARS-CoV-2 challenges and blocks the transmission of Omicron variant between hamsters. PoLixNano also displays versatile therapeutic potential in lung carcinoma and cystic fibrosis models. Our study provides new insights for designing delivery platforms of aerosol-inhaled mRNA therapeutics with clinical translation potential.
-
Genetics
Camouflaging attenuated Salmonella by cryo-shocked macrophages for tumor-targeted therapy.
In Signal Transduction and Targeted Therapy on 10 January 2024 by Wu, L., Du, Z., et al.
Live bacteria-mediated antitumor therapies mark a pivotal point in cancer immunotherapy. However, the difficulty in reconciling the safety and efficacy of bacterial therapies has limited their application. Improving bacterial tumor-targeted delivery while maintaining biosafety is a critical hurdle for the clinical translation of live microbial therapy for cancer. Here, we developed "dead" yet "functional" Salmonella-loaded macrophages using liquid nitrogen cold shock of an attenuated Salmonella typhimurium VNP20009-contained macrophage cell line. The obtained "dead" macrophages achieve an average loading of approximately 257 live bacteria per 100 cells. The engineered cells maintain an intact cellular structure but lose their original pathogenicity, while intracellular bacteria retain their original biological activity and are delay freed, followed by proliferation. This "Trojan horse"-like bacterial camouflage strategy avoids bacterial immunogenicity-induced neutrophil recruitment and activation in peripheral blood, reduces the clearance of bacteria by neutrophils and enhances bacterial tumor enrichment efficiently after systemic administration. Furthermore, this strategy also strongly activated the tumor microenvironment, including increasing antitumor effector cells (including M1-like macrophages and CD8+ Teffs) and decreasing protumor effector cells (including M2-like macrophages and CD4+ Tregs), and ultimately improved antitumor efficacy in a subcutaneous H22 tumor-bearing mouse model. The cryo-shocked macrophage-mediated bacterial delivery strategy holds promise for expanding the therapeutic applications of living bacteria for cancer.© 2023. The Author(s).
-
FC/FACS
-
Mus musculus (House mouse)
-
Cancer Research
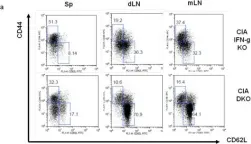
In PLoS One on 25 April 2013 by Lee, J., Lee, J., et al.
Fig.3.A

-
FC/FACS
-
Collected and cropped from PLoS One by CiteAb, provided under a CC-BY license
Image 1 of 1