Tyrosine kinase 2 (TYK2)-dependent cytokine signalling is integral to the pathogenesis of psoriasis. While BMS-986165, a highly selective TYK2 inhibitor, has recently been approved for oral treatment of psoriasis, its therapeutic potential via topical application remains unexplored.
We aim to investigate the efficacy of topically applying TYK2 inhibitor in psoriasis and to elucidate the underlying mechanisms driving the therapeutic effects of this delivery approach.
1.5% BMS-986165 ointment was applied topically to the back skin of imiquimod (IMQ)-induced psoriatic mice. To identify potential target cells influenced by the topical TYK2 inhibitor, we performed single cell RNA sequencing (scRNA-seq) and flow cytometry on mouse lesions. The role of TYK2 in vitro was assessed by silencing its expression or administering BMS-986165 in human keratinocytes (KCs). Mechanistic insights into TYK2 function in KCs were further investigated using RNA-seq, dual luciferase reporter assay and ChIP-qPCR.
External use of 1.5% BMS-986165 ointment significantly ameliorated the IMQ-induced psoriasis-like dermatitis. Importantly, topical TYK2 inhibitor attenuated proinflammatory capability of KCs. In vitro, TYK2 inhibition suppressed the transcription of nerve growth factor receptor (NGFR) by disrupting the AKT-SP1 signalling pathway. This impairment hindered the activation of activator protein 1 (AP1), thereby weakening the proinflammatory potential of KCs.
This study reveals a novel therapeutic potential for selective TYK2 inhibitor in topical manner on psoriasis therapy, which might prompt the development of topical treatment for psoriasis. Crucially, our findings provide an underexplored regulatory mechanism of TYK2 inhibitor in psoriasis.
Topical TYK2 inhibitor alleviates psoriasis-like dermatitis. Topical TYK2 inhibitor reduces psoriasis progression through restraining the inflammatory responses of keratinocytes. The inhibition of TYK2 regulates the inflammatory response of keratinocytes through AKT-SP1-NGFR-AP1 pathway.
© 2025 The Author(s). Clinical and Translational Medicine published by John Wiley & Sons Australia, Ltd on behalf of Shanghai Institute of Clinical Bioinformatics.
Product Citations: 66
In Clinical and Translational Medicine on 1 March 2025 by Fang, Z., Jiang, R., et al.
In International Journal of Biological Sciences on 15 April 2024 by Zhao, J., Chen, Y., et al.
Cysteinyl leukotriene receptor 1 (CYSLTR1) is observed to increase in psoriatic skin lesions. Montelukast, a CYSLTR1 antagonist, effectively treats inflammatory disorders, such as rheumatoid arthritis, multiple sclerosis, and atopic dermatitis. Thus, blocking CYSLTR1 may be a promising strategy for psoriasis immunotherapy. We prepared a montelukast sodium cream and solution and investigated their effects on psoriasis-like skin lesions induced by imiquimod (IMQ). After the treatment, serum, skin, and spleen samples were collected for evaluation. We treated human T helper (Th) 17 cells with montelukast in vitro to study its effect on Th17 differentiation and nuclear factor kappa-B (NF-κB) signaling. We also created a keratinocyte proliferation model induced by M5 cytokines and assessed the influence of montelukast on key psoriasis-related genes. We induced psoriasis in CYSLTR1 knockout (KO) mice using IMQ to explore the role of CYSLTR1 in psoriasis development. Montelukast sodium cream and solution effectively reduced the psoriasis area and severity index (PASI) and alleviated disease symptoms in IMQ-induced mice. Furthermore, reduced infiltration of inflammatory cells (Th1, Th17, and T follicular helper [Tfh] cells), decreased mRNA expression of cytokines in the skin (interleukin [IL]-17/F and IL-23), and lower serum concentrations of various cytokines (IL-2, IL-6, IL-13, and IL-17A/F) were observed. Montelukast cream and solution also decreased spleen size and the proportion of Th17 and Tfh cells, and significantly inhibited NF-κB signaling-related genes after application. Moreover, montelukast inhibited Th17 cell differentiation and suppressed NF-κB signaling in vitro. CYSLTR1 KO mice induced with IMQ showed improvement in PASI scores, serum IL-17A/F levels, and lower Th1 and Th17 cells in the spleen and skin compared to wild-type mice. Montelukast also suppressed the proliferation and inflammatory response of keratinocytes by regulating NF-κB signaling. Collectively, our results strongly indicate that inhibition of CYSLTR1 signaling to target the Th17 response holds significant promise as a therapeutic approach to manage psoriasis.
© The author(s).
-
Mus musculus (House mouse)
In Bio-protocol on 20 February 2024 by Aoudi, A., Labiad, O., et al.
Inflammatory bowel disease (IBD) is characterized by an aberrant immune response against microbiota. It is well established that T cells play a critical role in mediating the pathology. Assessing the contribution of each subset of T cells in mediating the pathology is crucial in order to design better therapeutic strategies. This protocol presents a method to identify the specific effector T-cell population responsible for intestinal immunopathologies in bone marrow-engrafted mouse models. Here, we used anti-CD4 and anti-CD8β depleting antibodies in bone marrow-engrafted mouse models to identify the effector T-cell population responsible for intestinal damage in a genetic mouse model of chronic intestinal inflammation. Key features • This protocol allows addressing the role of CD4+ or CD8αβ+ in an engrafted model of inflammatory bowel disease (IBD). • This protocol can easily be adapted to address the role of other immune cells or molecules that may play a role in IBD.
©Copyright : © 2024 The Authors; This is an open access article under the CC BY-NC license.
-
Mus musculus (House mouse)
-
Immunology and Microbiology
In Scientific Reports on 21 October 2023 by Van Damme, K. F. A., Hertens, P., et al.
A20 serves as a critical brake on NF-κB-dependent inflammation. In humans, polymorphisms in or near the TNFAIP3/A20 gene have been linked to various inflammatory disorders, including systemic lupus erythematosus (SLE) and rheumatoid arthritis (RA). Experimental gene knockout studies in mice have confirmed A20 as a susceptibility gene for SLE and RA. Here, we examine the significance of protein citrullination and NET formation in the autoimmune pathology of A20 mutant mice because autoimmunity directed against citrullinated antigens released by neutrophil extracellular traps (NETs) is central to the pathogenesis of RA and SLE. Furthermore, genetic variants impairing the deubiquitinase (DUB) function of A20 have been shown to contribute to autoimmune susceptibility. Our findings demonstrate that genetic disruption of A20 DUB function in A20 C103R knockin mice does not result in autoimmune pathology. Moreover, we show that PAD4 deficiency, which abolishes protein citrullination and NET formation, does not prevent the development of autoimmunity in A20 deficient mice. Collectively, these findings provide experimental confirmation that PAD4-dependent protein citrullination and NET formation do not serve as pathogenic mechanisms in the development of RA and SLE pathology in mice with A20 mutations.
© 2023. Springer Nature Limited.
-
Mus musculus (House mouse)
-
Pathology
In Immunity on 12 September 2023 by Larange, A., Takazawa, I., et al.
Ligation of retinoic acid receptor alpha (RARα) by RA promotes varied transcriptional programs associated with immune activation and tolerance, but genetic deletion approaches suggest the impact of RARα on TCR signaling. Here, we examined whether RARα would exert roles beyond transcriptional regulation. Specific deletion of the nuclear isoform of RARα revealed an RARα isoform in the cytoplasm of T cells. Extranuclear RARα was rapidly phosphorylated upon TCR stimulation and recruited to the TCR signalosome. RA interfered with extranuclear RARα signaling, causing suboptimal TCR activation while enhancing FOXP3+ regulatory T cell conversion. TCR activation induced the expression of CRABP2, which translocates RA to the nucleus. Deletion of Crabp2 led to increased RA in the cytoplasm and interfered with signalosome-RARα, resulting in impaired anti-pathogen immunity and suppressed autoimmune disease. Our findings underscore the significance of subcellular RA/RARα signaling in T cells and identify extranuclear RARα as a component of the TCR signalosome and a determinant of immune responses.
Copyright © 2023 Elsevier Inc. All rights reserved.
-
Mus musculus (House mouse)
-
Immunology and Microbiology
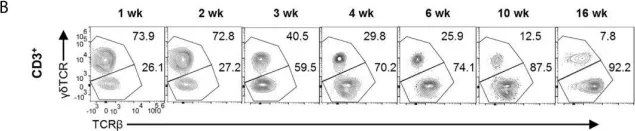
In Front Immunol on 13 August 2021 by Jing, Y., Cao, M., et al.
Fig.2.B

-
FC/FACS
-
Mus musculus (House mouse)
Collected and cropped from Front Immunol by CiteAb, provided under a CC-BY license
Image 1 of 3
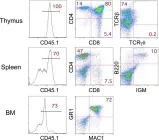
In Stem Cell Reports on 10 November 2015 by Ikawa, T., Masuda, K., et al.
Fig.2.C

-
FC/FACS
-
Mus musculus (House mouse)
Collected and cropped from Stem Cell Reports by CiteAb, provided under a CC-BY license
Image 1 of 3
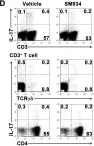
In PLoS One on 6 March 2012 by Hou, L. F., He, S. J., et al.
Fig.6.D

-
FC/FACS
-
Mus musculus (House mouse)
Collected and cropped from PLoS One by CiteAb, provided under a CC-BY license
Image 1 of 3